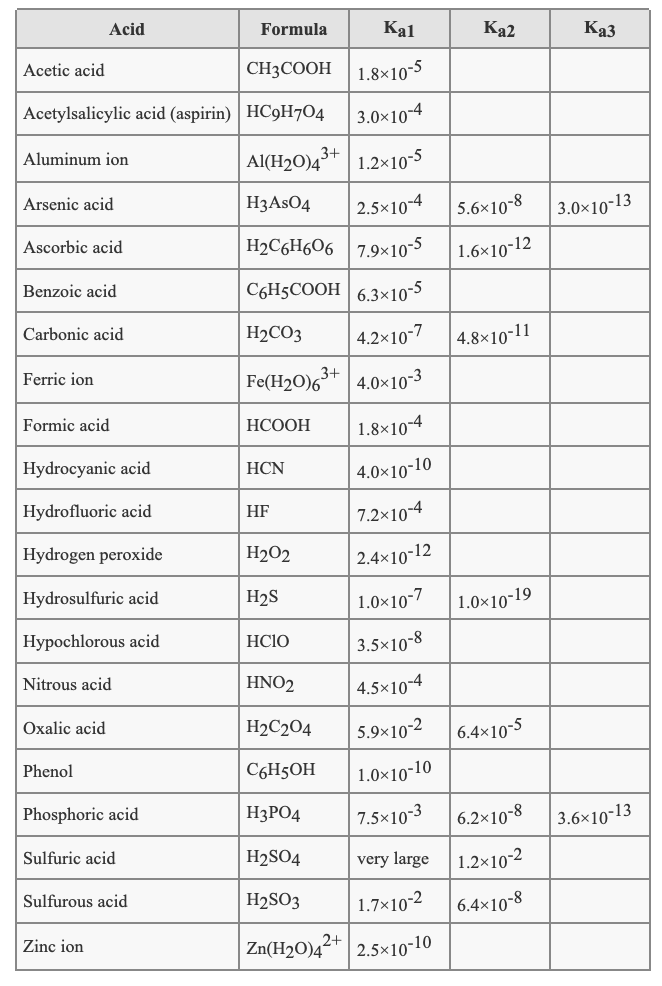
Ka Acid Name Formula Large Perchloric acid HClO4 3.2 * 109 Hydroiodic acid HI 1.0 * 109 Hydrobromic acid HBr
| Ka | Acid | |
|---|---|---|
| Name | Formula | |
| Large | Perchloric acid | HClO4 |
| 3.2 * 109 | Hydroiodic acid | HI |
| 1.0 * 109 | Hydrobromic acid | HBr |
What is the Ka value of HClO?
Apr 28, 2020 · Ka Acid Name Formula Large Perchloric acid HClO4 3.2 * 109 Hydroiodic acid HI 1.0 * 109 Hydrobromic acid HBr
Why pKa of HClO4 is more than HClO4?
Acid HA A-Ka pKa Acid Strength Conjugate Base Strength Hydroiodic HI I-Hydrobromic HBr Br-Perchloric HClO4 ClO4-Hydrochloric HCl Cl-Chloric HClO3 ClO3-Sulfuric (1) H2SO4 HSO4-Nitric HNO3 NO3-Strong acids completely dissociate in aq solution (Ka > 1, pKa < 1).
How to determine the conjugate base of HClO4?
Oct 29, 2021 · This particular acid is as strong of its ionizing properties in water. Hypochlorous acid, acid chloric acid. August 4 2012, a comment. Strong Acids are on the top left hand corner of the and have Ka > 1 2. Strong acids are that able to in water completely. Acids with values of than one considered weak. Hclo4 is the formula Perchloric acid.
What is the formula for HClO4?
Nov 21, 2021 · What is the ka for 0.03M Solution of HClO4 for 1.90 PH value. A) 7.2x10^-3 B) 8.27x10^-3 C) 8.27x10^-4 D) 6.27x10^-4 (I keep getting 5x10^-3 when I round all the values to 2 decimal places but it’s not in the options)
How do you find the Ka value?
As noted above, [H3O+] = 10-pH. Since x = [H3O+] and you know the pH of the solution, you can write x = 10-2.4. It is now possible to find a numerical value for Ka. Ka = (10-2.4)2 /(0.9 - 10-2.4) = 1.8 x 10-5.Mar 23, 2018
Is HClO4 a strong acid?
The 7 common strong acids are: HCl, HBr, HI, HNO3, HClO3, HClO4 and H2SO4 (1st proton only).
How do you find Ka of HCl?
0:074:48Find the Ka of an acid (Given pH) (0.1 M Hypochlorous acid) EXAMPLEYouTubeStart of suggested clipEnd of suggested clipWhich is each of the concentration of each of the products of the reaction divided by theMoreWhich is each of the concentration of each of the products of the reaction divided by the concentration of the reactants in this case is just HCl oh and the these are concentrations at equilibrium.
What is Ka of an acid?
The acid dissociation constant (Ka) is used to distinguish strong acids from weak acids. Strong acids have exceptionally high Ka values. The Ka value is found by looking at the equilibrium constant for the dissociation of the acid. The higher the Ka, the more the acid dissociates.
Is HClO4 Bronsted Lowry acid?
HClO4 act as Bronsted-Lowry acid as it donates the proton and forms a conjugate base. NH3 acts as a Bronsted-Lowry base as it accepts the proton from HClO4 and forms a Conjugate acid.
Why is HClO4 an acid?
Perchloric acid is a mineral acid with the formula HClO4. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid....Perchloric acid.NamesRelated compoundsHydrochloric acid Hypochlorous acid Chlorous acid Chloric acid46 more rows
What is the Ka of H2O?
Since [H2O] in pure water is 55.5 M, Ka = 1.8 x 10E-16, or pKa = 15.7 .
What is the Ka value of nh4cl?
As, other data, dissociation constant (Ka) of NH4Cl is 5.55 * 10-10mol dm-3. Now, these equilibrium concentrations of compounds can be substituted in dissociation equation as below. Because NH4+ is a weak acid, it's dissociation is small.
What is the Ka of HBr?
1.0 * 109KaAcid1.0 * 109Hydrobromic acidHBr1.3 * 106Hydrochloric acidHCl1.0 * 103Sulfuric acidH2SO42.4 * 101Nitric acidHNO328 more rows
How do you write Ka expression?
0:001:22Write Ka Expression 003 - YouTubeYouTubeStart of suggested clipEnd of suggested clipOkay so let's write the equilibrium constant expression for this particular problem. So how do we doMoreOkay so let's write the equilibrium constant expression for this particular problem. So how do we do this what is the equilibrium constant expression or for this one it since it's a weak acid we call
What is Ka acid base?
The acid dissociation constant (Ka) is a quantitative measure of the strength of an acid in solution while the base dissociation constant (Kb) is a measure of basicity—the base's general strength. Ka and pKa. Acids are classified as either strong or weak, based on their ionization in water.
How do you find the Ka value of acids?
Ka=([H+][A−]HA) where [H+],[A−]&[HA] are molar concentrations of hydronium ion, conjugate base and weak acid at equilibrium.Jul 10, 2017
What is the king of acids?
The king of acids is HNO3+3 HCl (aquea regia) because it dissolves gold and platinum. H2SO4 is Sulfuric Acid, no big deal). Perchloric acid (HClO4) is rated as highly corrosive especially at elevated temperatures and used to make rocket fuel.
Which is stronger, perchloric acid or oxyacid?
Perchloric acid is stronger than Periodic acid. While comparing acidic strengths in oxyacids, the larger the oxidation state of the central atom the stronger the acid is and if the oxidation states are same the more electronegative the central atom the stronger the acid is. 20.9K views. ·. View upvotes.
Why is H2SO4 and HClO4 so strong?
:H2SO4 and HClO4,HClO4 is strong because the perchlorate ion form by the removal of hydrogen atom is more stabilised than sulfate ion or in othr words the negtive charge in perchlorate ion is more dispersed as compared to sulfate ion. Sponsored by Grammarly. Fast.
What does ka mean in chemistry?
Ka is defined by the following equation. Simply Ka value indicates how easily the acid loses a proton. If Pka value is high then the acid is weak while Pka value low means the stronger acid. Pka value indicates the strength of the acid.
Which acid has a positive pKa?
Weak acids like carbonic acid , acetic acid etc. have highly positive pKa values, as they are only partially ionised in water. The ability on an acid to release H+ ion is also closely linked to the stability of the resultant conjugate base (A-) of the ionisation process.
What is the ability of an acid to release H+ ion?
The ability on an acid to release H+ ion is also closely linked to the stability of the resultant conjugate base (A-) of the ionisation process. The stabler the conjugate base, the stronger will be the acid. Continue Reading.
Is perchloric acid nucleophilic?
There are two numbers given with references, but both suggest that Perchloric Acid is an extremely strong acid. It provides strong acidity with minimal interference because perchlorate is weakly nucleophilic (explaining the high acidity of HClO4).”. reference: Perchloric acid - Wikipedia.