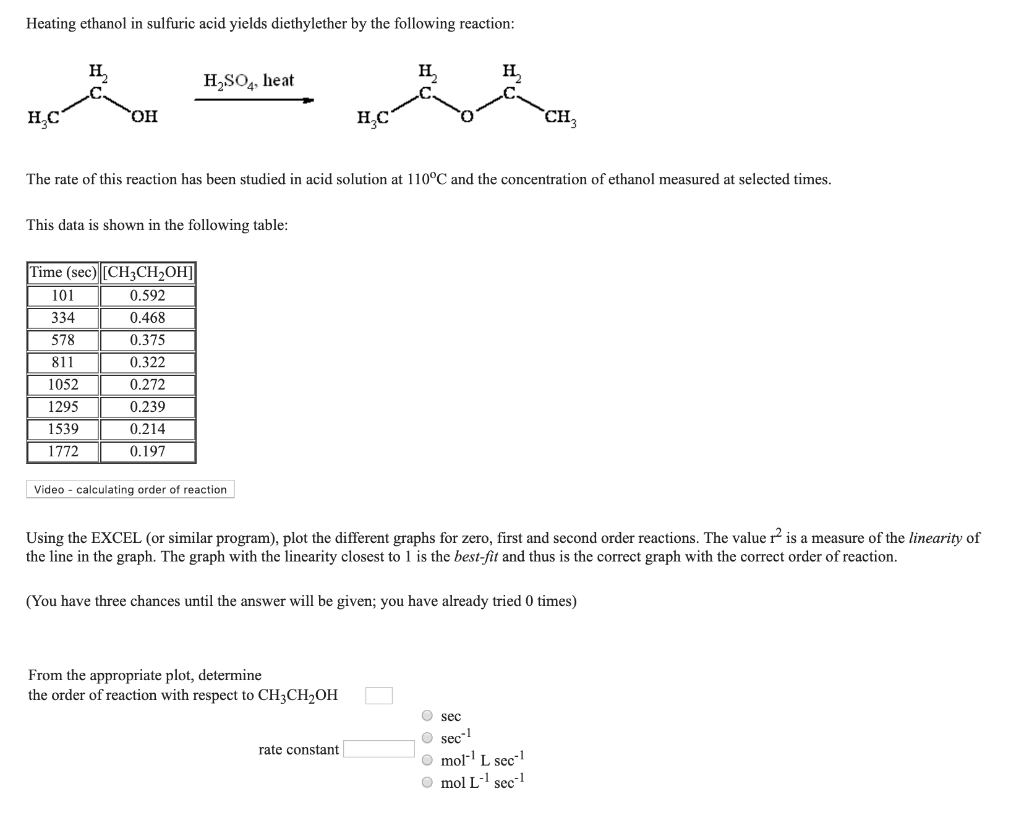
| 50 mL + 50 mL | ∆T solution | ∆Hrxn (kJ/mol) |
|---|---|---|
| 1 M HCl + 1 M NaOH | +6.7°C | -56 kJ/mol |
| 3 M HCl + 3 M NaOH | +20°C | -56 kJ/mol |
What is the balanced equation for HCl and NaOH?
What is the balanced equation for HCl and NaOH? HCl + NaOH = NaCl + H2O – Chemical Equation Balancer. What happens when HCl is neutralized by NaOH? The reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) is a neutralization reaction which results in the formation of a salt, sodium chloride (NaCl) , and water (H2O) .
What happens to HCL and NaOH when reacted?
- One mole of HCl would be fully neutralized by one mole of NaOH.
- Now two moles of HCl would be required to neutralize one mole of Ba (OH) 2 .
- We can then set the moles of acid equal to the moles of base.
Which solution is more concentrated, HCl or NaOH?
- Many experts have given answers for this. In their opinion Molar Solution (M) will be slightly more concentrated than molal (m) Solution. ...
- Carbon tetrachloride (CCl4)
- Chloroform (CHCl3) ,
- then their densities are 1.59 and 1.49 respectively. ...
- In the case of CC
Is heat generated with NaOH plus HNO3 reaction?
This reaction is classified as an exothermic reaction. The reaction of HCl(aq), a strong acid, with NaOH(aq), a strong base, is an exothermic reaction.
What is the specific heat of a solution of aqueous hydrochloric acid?
How does a chemical reaction change temperature?
How does heat transfer into a solution?
What is the initial temperature of a solution of 3.0 M hydrochloric acid and 3.0 M sodium hydro?
What is the energy released by a reaction?
Is heat kinetic energy?
Is 3.0 M NaOH corrosive?
See more
About this website
Why does HCl and NaOH exothermic?
The reaction is exothermic because both HCl and NaOH are trying to balance each other out. The reaction gives out energy. At first, this energy heats up the reacting mixture. Then heat is transferred from the mixture to its surroundings, and then the mixture cools to room temperature.
What is the reaction of HCl and NaOH?
Reactions of Acids and Bases A salt is a neutral ionic compound. Let's see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide. The overall equation for this reaction is: NaOH + HCl → H2O and NaCl.Jul 3, 2019
What is the net ionic equation when HCl and NaOH are mixed together?
1:503:15How to Write the Net Ionic Equation for HCl + NaOH = NaCl + H2OYouTubeStart of suggested clipEnd of suggested clipPlus plus the CL minus and in the water we said that's a liquid that stays together so that's justMorePlus plus the CL minus and in the water we said that's a liquid that stays together so that's just going to be h2o you resize this and this is the complete ionic equation for HCl plus na.
What is the net ionic equation of HCl and NaOH?
2:245:35NaOH + HCl - Sodium Hydroxide & Hydrochloric Acid - Net Ionic EquationYouTubeStart of suggested clipEnd of suggested clipWe need to do something first and that is we need to write the total ionic equation and here's howMoreWe need to do something first and that is we need to write the total ionic equation and here's how we're gonna do that everything that is in the aqueous phase that is soluble in water we're gonna
What is the heat of reaction for HCl and NaOH? - AskingLot.com
Click to see full answer. Then, what is the heat of neutralization of HCl and NaOH? The heat of reaction of one mole of H+ and OH- is 57.3 KJ. So, the heat of neutralisation of HCl and NaOH will be very cery close to 57.3 KJ per mole( As Both HCl and NaOH are strong elctrolytes so both of them quite easily without any considerable expense of energy furnish H+ and OH- ions respectively.
What is the enthalpy of neutralization of HCl and NaOH?
For example, one source which gives the enthalpy change of neutralisation of sodium hydroxide solution with HCl as -57.9 kJ mol-1, gives a value of -56.1 kJ mol-1 for sodium hydroxide solution being neutralised by ethanoic acid.
HCl + NaOH = NaCl + H2O - Chemical Equation Balancer
Reaction Type. Double Displacement (Acid-Base) List of Acids HCl List of Bases NaOH Net Ionic Equation. HCl + NaOH = NaCl + H2O might be an ionic equation.
Chemical Reactions Calculator - Symbolab
Free Chemical Reactions calculator - Calculate chemical reactions step-by-step
Chemical Reaction Calculator - Free online Calculator
Learn how to use the chemical reaction calculator with the step-by-step procedure at BYJU’S. For more calculators, register with us to get the solutions in a fraction of seconds
How does a chemical reaction change temperature?
Students have difficulty distinguishing the terms temperature and heat. Students have difficulty with the idea that the bulk material they can see is NOT the chemical reaction. A chemical reaction has no mass, has no specific heat, and does not change temperature. A chemical reaction consists of bonds breaking and bonds forming and this is a form of potential energy. In this demonstration, the chemical reaction releases heat to the immediate the surroundings. The water and dissolved chemicals gain heat - heat is transferred into the solution, which is mostly water. gained heat. When heat is transferred into the surroundings, the solution, from the chemical reaction, the solution increases in temperature. The water molecules being formed by the reaction have higher kinetic energy compare to the original water molecules in the solution. The newly formed water molecules collide with the original water molecules causing some of the original water molecules to move faster, there is a net increase in kinetic energy of the water molecules.
What is the initial temperature of a solution of 3.0 M hydrochloric acid and 3.0 M sodium hydro?
Equal volumes, 50.0 mL, of 3.0 M hydrochloric acid and 3.0 M sodium hydroxide solutions having an initial temperature of 20.0°C react in a calorimeter. The resultant solution records a temperature of 40.0°C. The heat gained by the resultant solution can be calculated using
What determines the heat exchanged at constant pressure?
Thermochemistry determine the heat exchanged at constant pressure,
What is the energy released by a reaction?
The energy released by the reaction is qreaction. By the law of conservation of energy:
Is 3.0 M NaOH corrosive?
The 3.0 M HCl solution is corrosive. The 3.0 M NaOH solution is caustic. Both the acid and base solutions can cause burns to exposed skin and damage to eyes. Use gloves and eye protection while preparing and performing the experiments.
Is heat kinetic energy?
Students have a difficult time understanding that through the vibration and movement of atoms and or molecules heat is exchanged and this is a form of kinetic energy.
Is HCl an exothermic reaction?
This demonstration is usually performed when topics in thermochemistry or thermodynamics are being discussed. The reaction of HCl(aq), a strong acid, with NaOH(aq), a strong base, is an exothermic reaction. The big idea for most calorimetry themed demonstrations is energy is conserved. Energy cannot be created or destroyed, but it can be exchanged.
How many KJ is the heat of reaction of H+ and OH-?
The heat of reaction of one mole of H+ and OH- is 57.3 KJ. So, the heat of neutralisation of HCl and NaOH will be very cery close to 57.3 KJ per mole ( As Both HCl and NaOH are strong elctrolytes so both of them quite easily without any considerable expense of energy furnish H+ and OH- ions respectively.
How to find the molar heat of neutralization?
Calculate the number of moles of base you add to determine the molar heat of neutralization, expressed using the equation ΔH = Q ÷ n, where "n" is the number of moles. For example, suppose you add 25 mL of 1.0 M NaOH to your HCl to produce a heat of neutralization of 447.78 Joules.
Is HCl and NaOH endothermic or exothermic?
Is the reaction between HCl and NaOH endothermic or exothermic? This reaction is classified as an exothermic reaction. The reaction of HCl(aq), a strong acid, with NaOH(aq), a strong base, is an exothermic reaction.
Do students have difficulty with the idea that the amount of reactants influences the heat exchanged?
Students do not have difficulty with the idea that the amount of reactants influences the heat exchanged, but they do have difficulty apply this concept.
Is HCl an exothermic reaction?
The reaction of HCl (aq), a strong acid, with NaOH (aq), a strong base, is an exothermic reaction. The big idea for most calorimetry demonstrations is energy is conserved. Energy cannot be created or destroyed, but it can be exchanged. qlost+ qgain = 0 or qreleased + qgain = 0.
Is heat exchanged proportional to the amount of moles of HCl and NaOH that react?
The heat exchanged is proportional to the amount of moles of HCl and NaOH that react. ∆H is reported as a per mole value. The value of ∆H is not influenced by the amount of reactants.
How to find the molar heat of neutralization?
Calculate the number of moles of base you add to determine the molar heat of neutralization, expressed using the equation ΔH = Q ÷ n, where "n" is the number of moles. For example, suppose you add 25 mL of 1.0 M NaOH to your HCl to produce a heat of neutralization of 447.78 Joules.
Is the neutralization of HCl and NaOH exothermic?
Furthermore, is the neutralization of HCl and NaOH exothermic? Heat of Neutralization: HCl(aq) + NaOH(aq) The reaction of HCl(aq), a strong acid, with NaOH(aq), a strong base, is an exothermic reaction. The big idea for most calorimetry themed demonstrations is energy is conserved. Energy cannot be created or destroyed, but it can be exchanged.
What is the specific heat of a solution of aqueous hydrochloric acid?
Since the solutions are mostly water, the solutions are assumed to have a density of 1.0 g/mL and a specific heat of 4.18 J/g°C. The reaction of an aqueous hydrochloric acid solution with an aqueous sodium hydroxide solution is represented by the neutralization chemical equation
How does a chemical reaction change temperature?
Students have difficulty distinguishing the terms temperature and heat. Students have difficulty with the idea that the bulk material they can see is NOT the chemical reaction. A chemical reaction has no mass, has no specific heat, and does not change temperature. A chemical reaction consists of bonds breaking and bonds forming and this is a form of potential energy. In this demonstration, the chemical reaction releases heat to the immediate the surroundings. The water and dissolved chemicals gain heat - heat is transferred into the solution, which is mostly water. gained heat. When heat is transferred into the surroundings, the solution, from the chemical reaction, the solution increases in temperature. The water molecules being formed by the reaction have higher kinetic energy compare to the original water molecules in the solution. The newly formed water molecules collide with the original water molecules causing some of the original water molecules to move faster, there is a net increase in kinetic energy of the water molecules.
How does heat transfer into a solution?
gained heat. When heat is transferred into the surroundings , the solution, from the chemical reaction, the solution increases in temperature.
What is the initial temperature of a solution of 3.0 M hydrochloric acid and 3.0 M sodium hydro?
Equal volumes, 50.0 mL, of 3.0 M hydrochloric acid and 3.0 M sodium hydroxide solutions having an initial temperature of 20.0°C react in a calorimeter. The resultant solution records a temperature of 40.0°C. The heat gained by the resultant solution can be calculated using
What is the energy released by a reaction?
The energy released by the reaction is qreaction. By the law of conservation of energy:
Is heat kinetic energy?
Students have a difficult time understanding that through the vibration and movement of atoms and or molecules heat is exchanged and this is a form of kinetic energy.
Is 3.0 M NaOH corrosive?
The 3.0 M HCl solution is corrosive. The 3.0 M NaOH solution is caustic. Both the acid and base solutions can cause burns to exposed skin and damage to eyes. Use gloves and eye protection while preparing and performing the experiments.