| ΔcH°gas (kJ/mol) | -1560.7 ± 0.3 |
| Method | Ccb |
| Reference | Pittam and Pilcher, 1972 |
| Comment | |
|---|---|
| Corresponding ΔfHºgas = -83.85 kJ/mol (simple calculation by NIST; no Washburn corrections); ALS | |
How do you calculate the enthalpy of combustion of ethane?
- Combustion - Boiler house topics - fuels like oil, gas, coal, wood - chimneys, safety valves, tanks - combustion efficiency
- Thermodynamics - Effects of work, heat and energy on systems
- Material Properties - Material properties for gases, fluids and solids - densities, specific heats, viscosities and more
What is the specific heat of ethane?
Witt, R.K.; Kemp, J.D. , The heat capacity of ethane from 15°K to the boiling point. The heat of fusion and the heat of vaporization , J. Am. Chem. Soc., 1937, 59, 273-276. [ all data ]
What is the balanced equation for the combustion of ethane?
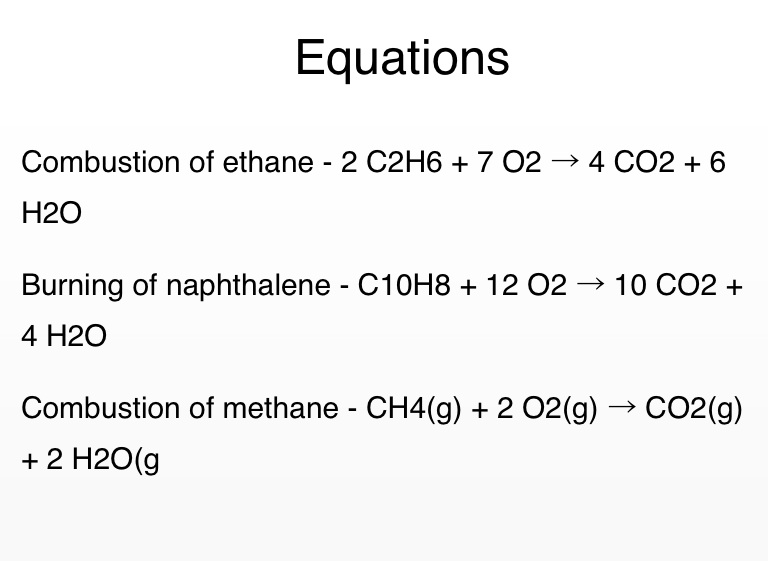
When the equation for combustion for ethane is balanced using integer coefficients, the ΔH for the reaction = -2834 kJ. The balanced chemical equation for the complete combustion of ethane is: 2 (C 2 H 6) + 7 (O 2) → 4 (CO 2) + 6 (H 2 O) + Heat Energy.
What is the enthalpy of CH4?
What is the enthalpy of the reaction CH4(g) + 4 Cl2(g) → CCl4(l) + 4 HCl(g)? −422.8 kJ. The enthalpy of fusion of solid n-butane is 4.66 kJ/mol. Calculate the energy required to melt 58.3 g of solid n-butane. 4.66 kJ. The highest recorded temperature in Africa was 55 °C in Kebili, Tunisia, on July 7, 1931. Convert this temperature to ...
What is the heat of combustion of ethane in kJ per gram?
What is the heat of combustion of ethane, in kJ per gram? 51.9 kJ/g.
Is the combustion of ethane exothermic?
The combustion of ethane (C2H4) is an exothermic reaction. C2H4(g) + O2(g) --> CO2(g) + H2O(g) ΔH= -1.39*103 kJ How much heat is given off when 4.79 g of C2H4 react with an excess of oxygen? Amita P.Mar 23, 2022
What is the combustion of ethane C2H6?
2C2H6(g)+7O2(g)⟶4CO2(g)+6H2O(g) How many moles of CO2 are produced when 5.95 mol of ethane is burned in an excess of oxygen? The combustion of ethane (C2H6) produces carbon dioxide and steam.Mar 14, 2021
What is the heat of formation of ethane C2H6?
Selected ATcT enthalpy of formation based on version 1.118 of the Thermochemical Network Species NameFormulaΔfH°(298.15 K)EthaneC2H6 (g)-83.75
Is combustion of ethane endothermic?
It is an endothermic reaction. Heat is required to break the chemical bonds between hydrogen and oxygen molecules of water. Here, energy is required to break the C-H bond in ethane to convert it to ethylene. Since energy is absorbed, this is an example of endothermic reaction.
How do you balance the combustion of ethane?
0:392:09Complete Combustion of Ethane (C2H6) Balanced Equation - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd 12 hydrogen's the 12 hydrogen's gets balanced on the right by putting a six here Co six timesMoreAnd 12 hydrogen's the 12 hydrogen's gets balanced on the right by putting a six here Co six times two gives me that 12.
Is ethane complete combustion?
As a hydrocarbon, it can undergo hydrocarbon combustion which gives off heat. Ethane is one of the hydrocarbon components of natural gas, which is a type of fossil fuel. In its purest form, ethane is a colourless, odourless substance....Properties.Chemical formulaC2H6Boiling Point-89oC3 more rows•Sep 27, 2021
What is the complete combustion of pentane?
C5H12+8O2→6H2O+5CO2.
Which equation represents incomplete combustion of ethane?
The equation below shows the incomplete combustion of ethene. C2H4(g)+2O2(g)→2CO(g)+2H2O(g) C 2 H 4 ( g ) + 2 O 2 ( g ) → 2 C O ( g ) + 2 H 2 O ( g ) .
Which is equation for ethane formation?
C2H6(g)→2C(s)+3H2.
How is ethane formed?
Ethane is also prepared by Wurtz reaction. When methyl bromide or methyl iodide and sodium are heated in the presence of dry ether ethane is formed.
How do you calculate heat of combustion?
How do you find the heat of combustion?Divide the number of moles of water vaporized by number of moles of fuel combusted.Find the product of heat of vaporization of water and the ratio of moles.Add the resultant to lower heating value of the fuel to obtain heat of combustion.Dec 1, 2021
What happens to ethane at low pressure?
However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The ethane phase diagram shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the ethane boiling point with changes in pressure.
Is ethane a gas?
Follow the links below to get values for the listed properties of ethane at varying pressure and temperature: Ethane is a gas at standard conditions. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The ethane phase diagram shows the phase behavior with changes in temperature and pressure.
How much heat is released from ethane?
The heat of combustion of ethane is 1560 kJ/mol. This amount of heat represents the energy released when a single mole of ethane is ignited in the presence of an excess of oxygen to produce two moles of carbon dioxide and three moles of water.
What does "heat of combustion" mean?
What does heat of combustion mean? Heat of combustion (ΔH°c) is the measure of the amount of energy released in the form of heat (q) when one mole of a substance is burned (combustion). The production of heat means the reaction is an exothermic process and gives off energy.
What is enthalpy in physics?
It is the sum of the internal energy added to the product of the pressure and volume of the system. It reflects the capacity to do non-mechanical work and the capacity to release heat.