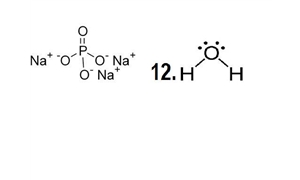
Trisodium phosphate
| PubChem CID | 24243 |
| Structure | Find Similar Structures |
| Chemical Safety | Laboratory Chemical Safety Summary (LCSS ... |
| Molecular Formula | Na3PO4 or Na3O4P |
| Synonyms | TRISODIUM PHOSPHATE Sodium phosphate 760 ... |
Full Answer
What is Na3PO4 in chemistry?
9 rows · Exact Mass: 163.92272832: Computed by PubChem 2.1 (PubChem release 2021.05.07) Monoisotopic ...
What is the solubility of Na3PO4?
4 rows · ›› Na3PO4 molecular weight. Molar mass of Na3PO4 = 163.940671 g/mol. This compound is also ...
What is the compound Na3PO4?
What is the formula weight of Na3PO4? 163.94 g/mol Click to see full answer Similarly, you may ask, how many grams are in Na3PO4? 163.940671 …
How do you calculate average molecular weight?
6 rows · Apr 23, 2019 · Molecular Weight/ Molar Mass: 163.94 g/mol: Boiling Point: 100 °C: Melting Point: 1,583 °C: ...
How do you calculate formula weight?
formula weight, in chemistry, a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a formula by the number of atoms of that element present in the formula, and then adding all of these products together.
What is the mass in grams of one formula unit of na3po4?
163.94 g/molOther names – Trisodium phosphate, Sodium orthophosphate, Tribasic sodium phosphateNa3PO4Sodium PhosphateDensity1.62 g/cm³Molecular Weight/ Molar Mass163.94 g/molBoiling Point100 °CMelting Point1,583 °C1 more row
What is the total mass of na3po4?
Therefore, the molar mass of sodium phosphate is 163.94 g/mol.
What is the mole of na3po4?
0:011:32How to Convert Moles of Na3PO4 to Grams - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we go to the periodic. Table and we find the mass for each element here we add all that up andMoreSo we go to the periodic. Table and we find the mass for each element here we add all that up and the molar mass of na3po4 is 163.94 and the units the units are grams per mole.
What is the molecular weight of nah2po4?
119.98 g/molMonosodium phosphate / Molar mass
What is the molar mass of Ca PO4 2?
0:021:30Molar Mass / Molecular Weight of Ca3(PO4)2: Calcium phosphateYouTubeStart of suggested clipEnd of suggested clipTimes 3 we end up with a molar mass for ca3po4 of 310.18 in the units.MoreTimes 3 we end up with a molar mass for ca3po4 of 310.18 in the units.
What element is Na3PO4?
The percent composition of Na3PO4, also known as trisodium phosphate is:Sodium 42.07 percent.Phosphorus 18.89 percent.Oxygen 39.04 percent.
What is the molecular weight of caco3?
100.0869 g/molCalcium carbonate / Molar mass
What is the formula for moles to grams?
To correctly estimate the number of moles, n , of a substance of a specific mass, m , (in grams), you need to follow the grams to moles formula: n = m / M , where, M is the molar mass of this material.Dec 2, 2020
How do I calculate moles?
How to find moles?Measure the weight of your substance.Use a periodic table to find its atomic or molecular mass.Divide the weight by the atomic or molecular mass.Check your results with Omni Calculator.Sep 8, 2021
What is the meaning of Na3PO4?
Sodium phosphateSodium phosphate (Na3PO4) sodiumphosphate. Sodium orthophosphate, tribasic.
How do you find the percent composition of Na3PO4?
Therefore the percentage composition of each element is:69164×100=42.1%Na.31164×100=18.9%P.64164×100=39%O.Feb 19, 2016
Is sodium phosphate an acid or a base?
Disodium hydrogen phosphate is a chemical compound with the formula Na2HPO4, also known as disodium phosphate. It’s more neutral (not acidic, or fu...
What is sodium phosphate used for in medicine?
Sodium biphosphate and sodium phosphate are sources of phosphorus which is a material that occurs naturally and is essential in every cell in the b...
What happens if your phosphate levels are low?
High phosphate levels seldom contribute to hypophosphateemia symptoms; rather symptoms generally arise from the underlying disorder that causes hyp...
What is sodium phosphate monobasic monohydrate?
Sodium Phosphate, Monobasic (monohydrate) is a reagent commonly used in molecular biology, biochemistry, and chromatography with very high bufferin...
What is the difference between sodium phosphate monobasic and dibasic?
Sodium phosphate monobasic has the chemical formula of NaH2PO4, and the chemical formula of Na2HPO4 has the sodium phosphate dibasic. As sodium pho...
How to find the formula weight?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find molar mass?
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
What is the difference between sodium phosphate monobasic and sodium phosphate dibasic?
What is the difference between sodium phosphate monobasic and dibasic? Sodium phosphate monobasic has the chemical formula of NaH2PO4, and the chemical formula of Na2HPO4 has the sodium phosphate dibasic.
What is the name of the compound that is made of hydrogen phosphate?
Disodium hydrogen phosphate is a chemical compound with the formula Na2HPO4, also known as disodium phosphate. It’s more neutral (not acidic, or fundamental). This is used to prevent food from clumping. It is made with phosphorus by reaction of some sodium hydroxide.
What is sodium phosphate used for?
Uses of Sodium Phosphate – Na 3 PO 4 1 Short-term, local treatment of inflammation with neomycin as bacterial prophylaxis. 2 Used after glaucoma surgery or after cataract surgery. 3 Used as a mild laxative, stimulates emptying of gall-bladder. 4 One of the most palatable of the saline laxatives. It is also used in the form of the oral solution (see below) as an antihypercal- cemic. 5 Used to control the pH of water hardness precipitation and control agent in mildly acidic solutions.
Is sodium phosphate a monohydrate?
Sodium Phosphate, Monobasic (monohydrate) is a reagent commonly used in molecular biology, biochemistry, and chromatography with very high buffering capacity. Monobasic sodium phosphate is extremely hygroscopic, and soluble in water.
Computing molar mass (molar weight)
To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
Computing molecular weight (molecular mass)
To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.
Definitions of molecular mass, molecular weight, molar mass and molar weight
Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12)
What is formula weight?
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Formula weights are especially useful in determining the relative weights ...
How to calculate the weight of an element?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. The atomic weights used on this site come from NIST, the National Institute ...
What is the formula for calculating molar mass?
If the formula used in calculating molar mass is the molecular formula , the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom ...
Computing molar mass (molar weight)
To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
Computing molecular weight (molecular mass)
To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.
Definitions of molecular mass, molecular weight, molar mass and molar weight
Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12)