What is the formula for specific enthalpy of moist air?
Specific enthalpy (enthalpy per kg of dry air) of moist air is the sum of the specific enthalpy of dry air and the specific enthalpy of the water vapor in the air. This is given by the equation: h = ha + H*hg; where ha is the specific enthalpy of dry air, H is the humidity ratio, and hg is the specific enthalpy of water vapor. 2
What is the chemical formula of the water component of moist air?
So H20 is the chemical formulae of the water component of moist air. O2 is the chemical formula of the oxygen component comprising moist air.
What is the specific volume of moist air?
Specific volume is defined as the total volume of humid air per mass unit of dry air 1 specific volume of moist air per mass unit of dry air, or 2 specific volume of moist air per mass unit of dry air and water vapor More ...
What is moist air?
Moist air is a mixture of dry air and water vapor. In atmospheric air water vapor content varies from 0 - 3% by mass. The enthalpy of moist and humid air includes the enthalpy of the dry air - the sensible heat
What is moist air chemical formula?
Answer. Moist air like dry air is a mixture. It has no chemical formula apart from the chemical formulae of its component parts. So H20 is the chemical formulae of the water component of moist air.
What makes moist air?
Humidity is the presence of water vapor in the atmosphere. The more water evaporates in a given area, the more water vapor rises into the air, and the higher the humidity of that area is. Hot places tend to be more humid than cool places because heat causes water to evaporate faster.
What solution is moist air?
Humid air is just a mixture of gases. It is not a solution (neither of a gas nor of a liquid) in the sense that a solvent (air) is needed to dissolve a solute (water). In this sense, water vapour is not dissolved in air; it is just another gas that is mixed with the other gases in the air.
Is moist air liquid or gas?
Water existing as a gas is called water vapor. When referring to the amount of moisture in the air, we are actually referring to the amount of water vapor. If the air is described as "moist", that means the air contains large amounts of water vapor.
What is moist air called?
humidityThe amount of water vapor in the air is called humidity.
What is the unit of humidity?
The specific humidity unit is the most reliable unit of measurement of humidity. This measures the weight of water vapor per unit weight of air and it is expressed as grams of water vapor per kilogram of air g.kg-1 is the specific humidity unit.
How do you calculate specific volume of moist air?
Moist Air - Density vs. Water Content and TemperatureDensity of Dry Air. The density of dry air can be calculated. ρa = pa / Ra T. ... Density of Water Vapor. The density of the water vapor can be calculated. ρw = pw / Rw T. ... Density of Moist Air - an Air Vapor Mixture. The amount of water vapor in air influences the density.
How is dew formed?
Dew forms as temperatures drop and objects cool down. If the object becomes cool enough, the air around the object will also cool. Colder air is less able to hold water vapor than warm air. This forces water vapor in the air around cooling objects to condense.
How is humidity ratio calculated?
Humidity ratio is measured in units of pound-mass of water per pound-mass of dry air. By multiplying by 7000 [grains per pound-mass], this value may be expressed in grains of water per pound-mass of dry air.
What is ideal gas equation for dry and moist air?
For dry air M = Md = 28.97g (you will do this in HW 2). For water vapor the ideal gas law is eαv = RvT. Mw = 18.016g and Rv = 461.51JK−1kg−1.
What is the density of moist air?
Density of moist air vs. pressure ranging 75 - 1000 mmHg.Density of Moist Air (kg/m3)Pressure (mm Hg)Temperature (oC)8251.3971.3488001.3551.3077751.3121.26638 more rows
What is dry air?
In atmospheric thermodynamics and chemistry, air that is assumed to contain no water vapor. Compare moist air. Generally, air with low relative humidity.
What is the chemical formula of H20?
It has no chemical formula apart from the chemical formulae of its component parts. So H20 is the chemical formulae of the water component of moist air. O2 is the chemical formula of the oxygen component comprising moist air
Is H20 a mixture or a mixture?
Moist air like dry air is a mixture. It has no chemical formula apart from the chemical formulae of its component parts. So H20 is the chemical formulae of the water component of moist air. O2 is the chemical formula of the oxygen component comprising moist air.
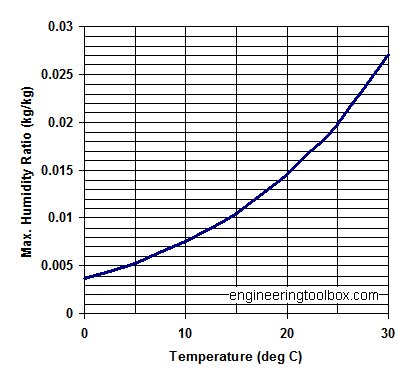
When air contains a maximum amount of moisture that it can hold at a particular temperature, the air is called?
Therefore when the air contains a maximum amount of moisture that it can hold at a particular temperature, the air is in a state of saturation and is called “saturated air”. The corresponding partial pressure of water vapor is referred as “partial pressure water vapor” by pv,s.
How is humidity measured?
Humidity is measured using instruments called hygrometers. The total pressure of humid air is equal to the sum of the partial pressures of its constituents, including water vapour. The partial pressure of water vapour in air, pw, depends on the molar concentration of water in the gas phase: (11.156) p w = y w p T.
What happens when air is saturated?
Air is said to be saturated with water vapour at a particular temperature and pressure if its humidity is the maximum it can be under those conditions. Addition of further water vapour to saturated air results in the condensation of liquid water in the form of droplets or a mist.
What is the temperature at which water becomes saturated?
If a mixture of water vapour in air is cooled, the temperature at which the mixture becomes saturated is called the dew point or saturation temperature. The dew point is the temperature at which pure water exerts a vapour pressure equal to the partial pressure of water vapour in the mixture. The relative humidity of air is defined as the ratio ...
Is dew point constant in seawater?
In addition, dew point depends on the moisture content in moist air only, which means td is constant when the moisture content is stable. In seawater desalination, the condensing temperature must be lower than the dew point if condensing fresh water from moist air.
How to find the specific enthalpy of dry air?
This is given by the equation: h = ha + H*hg; where ha is the specific enthalpy of dry air, H is the humidity ratio, and hg is the specific enthalpy of water vapor.
What is humidity ratio?
Examine H. Humidity ratio is the ratio of the mass of water vapor in the moist air and the mass of dry air. In layman's terms, it gives the wetness of air. Along with the temperature of air, this must be given in the problem.
What is the study of air conditioning?
Psychrometry is the study of properties of air, which is an important part of the field of Air-conditioning. One important property is the enthalpy, which is a measure of the energy present in the substance at a certain state.
How to find the mass of water vapor?
The mass of the water vapor can be calculated by using the humidity ratio and the mass of air as
What is specific volume?
Specific volume is defined as the total volume of dry air and water vapor mixture per kg of dry air and water vapor (SI-units). The specific volume can be expressed as:
What is the relative humidity of water?
The Relative Humidity (RH) is the ratio of the actual water vapour pressure to the saturation water vapour pressure at the prevailing temperature.
How does unsaturated air evaporate water?
Unsaturated air evaporates water from the wet wick. The heat required to evaporate the water into the air stream is taken from the air stream, which cools in contact with the wet surface, thus cooling the thermometer beneath it. An equilibrium wet surface temperature is reached which is very roughly half way between ambient temperature and dew point temperature.
How to measure dew point?
The dew point can also be measured directly by cooling a mirror until it fogs. The RH is then given by the ratio
Sensible and latent heat of moist air
Moist air is a mixture of dry air and water vapor. In atmospheric air water vapor content varies from 0 - 3% by mass. The enthalpy of moist and humid air includes the
Engineering ToolBox - SketchUp Extension - Online 3D modeling!
Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with the amazing, fun and free SketchUp Make and SketchUp Pro .Add the Engineering ToolBox extension to your SketchUp from the SketchUp Pro Sketchup Extension Warehouse!.
Privacy
We don't collect information from our users. Only emails and answers are saved in our archive. Cookies are only used in the browser to improve user experience.
Advertise in the ToolBox
If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. You can target the Engineering ToolBox by using AdWords Managed Placements.
Citation
Engineering ToolBox, (2004). Moist Air - Enthalpy . [online] Available at: https://www.engineeringtoolbox.com/enthalpy-moist-air-d_683.html [Accessed Day Mo. Year].
What is xs in air?
xs = humidity ratio of the air at saturation at the same temperature and pressure (kgh20/kgdry_air)
What is degree of saturation?
Degree of Saturation is the ratio of the humidity ratio of moist air - to the humidity ratio of saturated moist air at the same temperature and pressure.
Engineering ToolBox - SketchUp Extension - Online 3D modeling!
Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with the amazing, fun and free SketchUp Make and SketchUp Pro .
Privacy
We don't collect information from our users. Only emails and answers are saved in our archive. Cookies are only used in the browser to improve user experience.
Advertise in the ToolBox
If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. You can target the Engineering ToolBox by using AdWords Managed Placements.
Citation
Engineering ToolBox, (2009). Moist Air - Properties. [online] Available at: https://www.engineeringtoolbox.com/moist-air-properties-d_1256.html [Accessed Day Mo. Year].
What are the two most dominant components of air?
Air is a mixture of several gases, where the two most dominant components in dry air are 21 vol% oxygen and 78 vol% nitrogen. Oxygen has a molar mass of 15.9994 g/mol and nitrogen has a molar mass of 14.0067 g/mol. Since both of these elements are diatomic in air - O2 and N2, the molar mass of oxygen gas is aprox. 32 g/mol and the molar mass of nitrogen gas is aprox. 28 g/mol.
How to find average molar mass?
The average molar mass is equal to the sum of the mole fractions of each gas multiplied by the molar mass of that particular gas: M mixture = (x 1 *M 1 + ......+ x n *M n) (1) where. x i = mole fractions of each gas. M i = the molar mass of each gas.
What is the molar mass of nitrogen?
Oxygen has a molar mass of 15.9994 g/mol and nitrogen has a molar mass of 14.0067 g/mol. Since both of these elements are diatomic in air - O2 and N2, the molar mass of oxygen gas is aprox. 32 g/mol and the molar mass of nitrogen gas is aprox. 28 g/mol.
How to find molecular weight?
The molecular weight (or molar mass) of a substance is the mass of one mole of the substance, and can be calculated by summarizing the molar masses of all the atoms in the molecule.
When does vapor saturate?
The vapor in air may saturate to droplets when temperature is decreased or pressure is increased.
Is water vapor always present in air?
Water vapor is almost always present in air. The content may vary and the maximum amount possible of water vapor in dry air depends on the temperature of the air. Water vapor - H2O - is composed of one oxygen atom and two hydrogen atoms. Hydrogen is the lightest element with a molar mass of 1 g/mol, while oxygen has 16 g/mol.