What is the formal charge on each atom in Co and Oh?
The formal charge of O in CO is +1. Refer to the structure. We can calculate it as follows: Formal charge= 6-2- (1/2 ×6) = +1. Similarly, what is the formal charge on each atom in OH? The formal charge is 6−6=0.
How do you determine formal charges for the atoms in CO2?
In order to determine formal charges for the atoms in the carbon dioxide molecule you need to take into account the fact that CO2 has three resonance structures that look like this:
What is the formal charge of carbon?
Since carbon has 4 valence electrons, its formal charge will be zero. Was this answer helpful? Thank you. Your Feedback will Help us Serve you better.
How do you find the formal charge of a compound?
For finding out the formal charge of an element in a compound, there is this formula: Formal charge= no: of valence e-s - no: of unshared e-s - 1/2× no: of shared e-s. The Lewis structure of CO is as follows: . Refer to the structure. We can calculate it as follows: Formal charge= 6-2-(1/2 ×6) = +1.
What is the charge of carbon in CO?
The oxidation state of carbon in carbon monoxide is +2 in each of these structures. It is calculated by counting all the bonding electrons as belonging to the more electronegative oxygen.
What is the formal charge on carbon in CO and respectively?
Answer: CO2 is a neutral molecule with 16 total valence electrons. Carbon double bonded to both oxygen atoms (carbon = 0, oxygens = 0, total formal charge =0).
What is the charge of CO in con?
Table of Common Element ChargesNumberElementCharge27cobalt2+, 3+28nickel2+29copper1+, 2+30zinc2+88 more rows•Dec 23, 2018
What is the formal charge of C in CO 32?
Now, we will find out the formal charge of the carbon atom. Therefore the formal charge of the carbon atom is 0. Hence the correct option is (D).
Why does CO have a formal charge?
- The oxygen formed a triple bond with carbon means oxygen has a positive charge on it. - Therefore the net formal charge on the carbon monoxide = - 1 + 1 = 0. - So the formal charge of carbon monoxide (CO) is zero. Note: We can count the formal charge of an individual atom and formal charge of a molecule also.
What is the charge of oxygen in CO?
0:311:33How to Calculate the Formal Charges for CO (Carbon Monoxide) - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd the oxygen. We have two four six of those four minus two is two two minus three that'll give usMoreAnd the oxygen. We have two four six of those four minus two is two two minus three that'll give us a negative one formal charge.
What is the oxidation number of CO?
Hence, the oxidation state of Co is +3.
Does CO ligand have a charge?
They occur as neutral complexes, as positively-charged metal carbonyl cations or as negatively charged metal carbonylates. The carbon monoxide ligand may be bound terminally to a single metal atom or bridging to two or more metal atoms.
Is CO neutral ligand?
Examples of common ligands are the neutral molecules water (H2O), ammonia (NH3), and carbon monoxide (CO) and the anions cyanide (CN-), chloride (Cl-), and hydroxide (OH-).
What is the structure of CO 32?
Lewis structure of CO32- (carbonate) ion In carbonate ion, there is two oxygen atoms which has -1 charge on each of them. One of these oxygen atom take a proton (H+ ion) and form a -OH group.
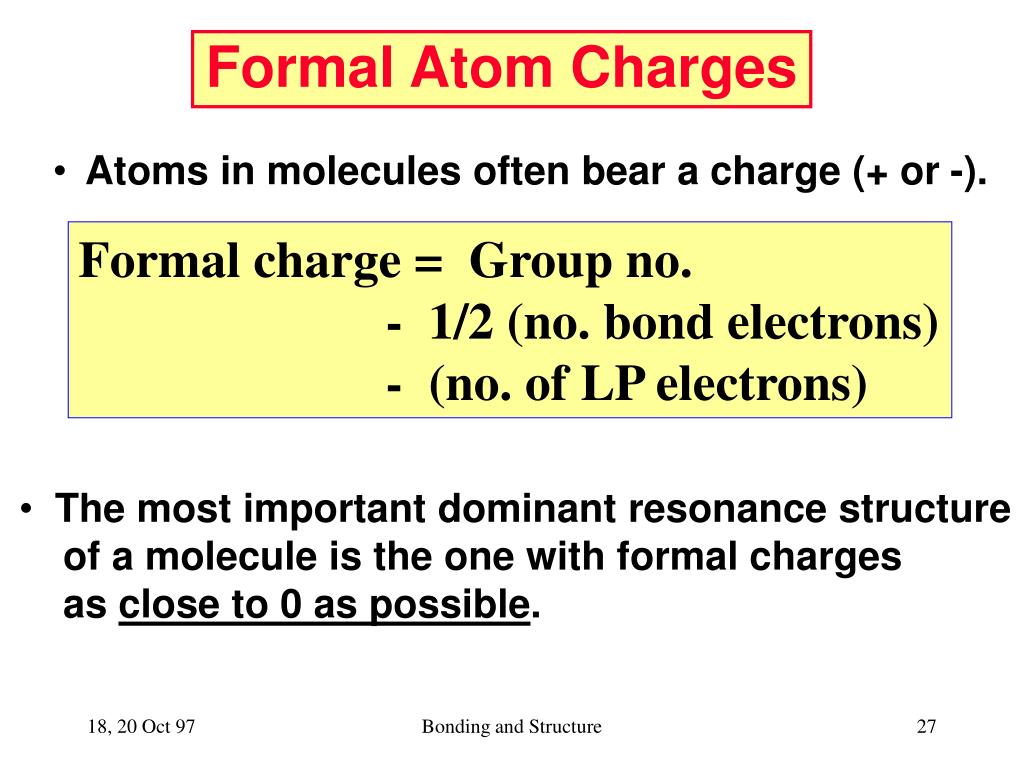
How do you find formal charge?
To find formal charges in a Lewis structure, for each atom, you should count how many electrons it "owns". Count all of its lone pair electrons, and half of its bonding electrons. The difference between the atom's number of valence electrons and the number it owns is the formal charge.
How many electrons are in two molecules of the ion co32 -?
Thirty-two electrons as required.
What is formal charge?
The formal charge over an atom of a polyatomic molecule or ion is the difference between the valence electron of that atom in the elemental state and the number of electrons assigned to that atom in Lewis structure.
How many valency does oxygen have?
Oxygen should have 6 as valency, but has 5 attached, Formal charge = 6-5 =+1. Net Formal Charge. +1 and -1 cancel for a net formal charge of zero.
What is formal charge?
Formal charge means ignore the lone pair of electrons and electrons shared by that atom from valence electron then whatever is left with you is signature of formal charge. That is, valence electron minus total lone pairs of electrons minus electrons shared by that atom only.
How many particles are in a mole of carbon?
We know that Carbon has atomic mass equal to 12g, I. E. One mole of carbon atom weighs 12g. We know that one mole of any substance contains 6.0225X10^23 particles (atoms, molecules, ions, etc.).
How many electrons does oxygen have?
The oxygen atom has 6 electrons on the last layer. In order to be stable, it needs 8 electrons. Since carbon monoxide is a stable molecule (as far as I know), it means the carbon provides two electrons, while the oxygen gets two electrons. Therefore, oxygen has a charge of -2 (excess of electrons) and this leaves carbon a charge of +2 ...
How much does carbon-12 have?
Carbon-12 has exact mass of 12.000 and it is easy to handle. We actually don't use carbon-12 whole as a reference. We use 1/12th part of carbon-12 isotope. First We divide the atom of Carbon-12 in 12 equal Parts. The part obtained is the 1/12th part of the total and has a exact mass of 1.000.
How many moles of an element have mass?
One mole of any element/compound has mass equal to its gram atomic/molecular mass.
Is electron density weighted toward oxygen?
You are correct in that electron density IS weighted toward the oxygen.
Is CO polarized or polarized?
CO is polarized, all right, but it’s polarized with the negative end at oxygen, as would be predicted by electronegativity. The formal charges in the Lewis structure of CO do give you a hand-waving explanation for the fact that it’s not nearly as polar as formaldehyde, which has no formal charges.