Types
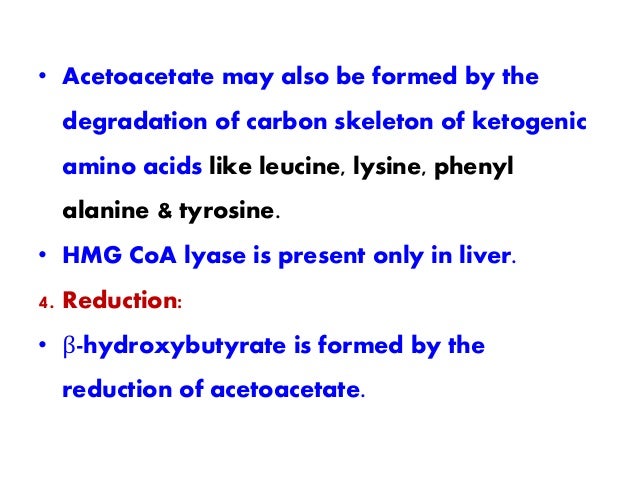
- Acetoacetate – the simplest beta-keto acid that is formed after metabolism of fat. It is the first ketone body produced by the liver.
- Beta-hydroxybutyrate – it is formed from acetoacetate. It is not technically a ketone body because of its structure. ...
- Acetone – it is the least abundant (2%) ketone body and is also termed as “fruity breath smell”. ...
Which is the first ketone in the ketone series?
Here, methanone or ethanone cannot exist as they cannot have a secondary carbonyl group, thus propanone is the first in the ketone series. The R and R’ can have any kind of alkyl group for the ketone series, but to find the first, basic hydrocarbons are put in their place.
How are the common names for ketones derived?
Common names for ketones are derived by naming each carbon group bonded to carbon as a separate word followed by the word “ketone.”
Where are ketone groups found in nature?
They are found in several sugars and in compounds for medicinal use, including natural and synthetic steroid hormones. Molecules of the anti-inflammatory agent cortisone contain three ketone groups. When an oxygen atom forms a double bond to a carbon atom, a carbonyl functional group is obtained.
What is the most important ketone?
The most important ketone is acetone (CH3COCH3), a liquid with a sweetish odour. Acetone is one of the few organic compounds that is infinitely soluble in water (i.e., soluble in all proportions); it also dissolves many organic compounds.
Why is ketone the first member of propanone?
Therefore in an organic compound with a ketone group there should be at least 3 carbon atoms so that the ketone group lies in between. Since methanone would only contain 1 carbon atom - no such compound exists, the first compound with a susbstitued ketone group is thus propanone.
What is the second member of ketone?
The name of second member of ketone series is Butanone.
What are the first five members of ketone?
methyl propyl ketone. methylpropyl ketone. methyl n-propyl ketone.
Who is the first member of ketone homologous series?
Methanone.
Who is the first member of aldehyde?
FormaldehydeFormaldehyde, is the first member of the aldehyde family is a at a ordinary temperature.
Which is the first member of alcohol?
MethanolMethanol CH3OH, also called methyl alcohol, wood alcohol, or wood spirit, the simplest of a long series of organic compounds called alcohols, consisting of a methyl group CH3 linked with a hydroxy group OH. Methanol was formally produced by the destructive distillation of wood.
What is ketone functional group?
A carbon double bonded to an oxygen is called a carbonyl group. Compounds in which the carbon of a carbonyl group is bonded to two other carbons are called ketones. Ketones are named the same way as are alkenes except that an -one ending is used.
What are examples of ketones?
Some Common Ketones. In terms of scale, the most important ketones are acetone, methylethyl ketone, and cyclohexanone. They are also common in biochemistry, but less so than in organic chemistry in general. Dimethyl ketone, CH3COCH3, commonly called acetone, is the simplest ketone.
How are ketones named?
They are named by finding the carbonyl group and identifying it with a location number, if necessary, then adding the suffix "-one." The common name for ketones is determined by naming the alkyl groups attached to the carbonyl (in alphabetical order), then adding 'ketone'.
What is the first member of Ester?
Among the given compounds, methyl methanoate is the first member of ester homologous series.
What is the homologous series of ketone?
A homologous series of ketones can include propanone, butanone, and 2-pentanone with chemical formulas of CH3COCH3, CH3CH2COCH3, and CH3CH2CH2COCH3, respectively where each successive compound differs from the previous one by a –CH2 group.
What is the first member of alkene?
etheneFor alkenes, first member is ethene followed by propene, butene etc.
What is second member of ketone write its formula?
H3C – CO – CH3. This is also called Acetone. Ketones are carbonyl compounds with the general formula R (CO) R′, where R and R′ are hydrocarbon radicals. 2nd member of ketone group is butanone (2 – Butanone) .
What is ketone group?
ketone, any of a class of organic compounds characterized by the presence of a carbonyl group in which the carbon atom is covalently bonded to an oxygen atom.
What are examples of ketones?
Some Common Ketones. In terms of scale, the most important ketones are acetone, methylethyl ketone, and cyclohexanone. They are also common in biochemistry, but less so than in organic chemistry in general. Dimethyl ketone, CH3COCH3, commonly called acetone, is the simplest ketone.
Which of the following is a ketone group?
Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.
What is the simplest ketone?
Acetone. In chemistry, a ketone / ˈkiːtoʊn / is a functional group with the structure R 2 C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl ), with the formula CH 3 C (O)CH 3. Many ketones are of great importance in biology ...
Where does the word "ketone" come from?
The word ketone is derived from Aketon, an old German word for 'acetone'. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix -ane of the parent alkane to -anone. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for ...
How does an aldehyde differ from a ketone?
Characterization. An aldehyde differs from a ketone in that it has a hydrogen atom attached to its carbonyl group, making aldehydes easier to oxidize. Ketones do not have a hydrogen atom bonded to the carbonyl group, and are therefore more resistant to oxidation.
What is the name of the compound that contains alkene and alkyne?
Ketones containing alkene and alkyne units are often called unsaturated ketones. The most widely used member of this class of compounds is methyl vinyl ketone, CH 3 C (O)CH=CH 2, which is useful in the Robinson annulation reaction. Lest there be confusion, a ketone itself is a site of unsaturation; that is, it can be hydrogenated .
What is the bond angle of a ketone?
Ketones are trigonal planar around the ketonic carbon, with C−C−O and C−C−C bond angles of approximately 120°.
What is the alphabetical order of alkyl groups?
When the two alkyl groups are the same, the prefix "di-" is added before the name of alkyl group.
Is ketone a nucleophile?
Thus, ketones are nucleophilic at oxygen and electrophilic at carbon. Because the carbonyl group interacts with water by hydrogen bonding, ketones are typically more soluble in water than the related methylene compounds. Ketones are hydrogen-bond acceptors.
What is the simplest ketone?
The simplest ketone, CH 3 COCH 3, whose IUPAC name is 2-propanone, is almost always called by its common name, acetone, which is derived from the fact that it was first prepared by heating the calcium salt of acetic acid.
How are cyclic ketones derived?
In cyclic ketones, numbering of the atoms of the ring begins with the carbonyl carbon as number 1. Common names for ketones are derived by naming each carbon group bonded to carbon as a separate word followed by the word “ketone.”.
Why are ketones reactive?
Ketones are highly reactive, although less so than aldehydes, to which they are closely related. Much of their chemical activity results from the nature of the carbonyl group. Ketones readily undergo a wide variety of chemical reactions. A major reason is that the carbonyl group is highly polar; i.e., it has an uneven distribution of electrons. This gives the carbon atom a partial positive charge, making it susceptible to attack by nucleophiles, which are species attracted to positively charged centres. Typical reactions include oxidation-reduction and nucleophilic addition. The polarity of the carbonyl group affects the physical properties of ketones as well.
Why is the reaction halted at the ketone stage?
The reaction can be halted at the ketone stage because ketones are generally resistant to further oxidation. Oxidation of a secondary alcohol to a ketone can be accomplished by many oxidizing agents, most often chromic acid (H 2 CrO 4 ), pyridinium chlorochromate (PCC), potassium permanganate (KMnO 4 ), or manganese dioxide (MnO 2 ).
How many ketone groups are in cortisone?
Molecules of the anti-inflammatory agent cortisone contain three ketone groups. When an oxygen atom forms a double bond to a carbon atom, a carbonyl functional group is obtained.
What chemical compound forms a double bond with a carbon atom?
chemical compound: Aldehydes and ketones. When an oxygen atom forms a double bond to a carbon atom, a carbonyl functional group is obtained. The carbon atom of a carbonyl group is... Only a small number of ketones are manufactured on a large scale in industry. They can be synthesized by a wide variety of methods, ...
How many carbon atoms are in a cyclic ketone?
The longest chain contains six carbon atoms and numbering of the carbon must begin at the end that gives the smaller number to the carbonyl carbon. The carbonyl group is on carbon 3, and the methyl group is on carbon 5. In cyclic ketones, numbering of the atoms of the ring begins with the carbonyl carbon as number 1.
What is the starting compound for a ketone?
Several reactions can be used to synthesize a ketone depending on the starting molecule including a Friedel-Crafts acylation, where the starting compound involves the use of an aromatic group and acyl chloride, an alkyne hydration reaction, where the starting compound is an alkyne compound, or host of other reactions.
What are the reactions that are used to synthesize ketone?
For a ketone, there are a number of reactions that can be used for synthesis. Reactions can be a Friedel-Crafts acylation where the starting compound involves the use of an aromatic group and acyl chloride, an alkyne hydration reaction where the starting compound is an alkyne compound, or host of other reactions.
How to tell if a ketone is aldehyde?
While aldehyde contains a hydrogen atom connected to its carbonyl group, ketone does not have a hydrogen atom attached. There are a few ways to know you are encountering a ketone. The first is by looking at the ending of the chemical word. If the suffix ending of the chemical name is '-one,' then you can be sure there is a ketone present in ...
Do ketones have high boiling points?
Ketones have high boiling points and love water (high water solubility). Let's dig a little deeper with the physical property of a ketone. The oxygen in a ketone absolutely loves to take all the electrons it can get its hands on.
Is a ketone a hydrogen atom?
More importantly, it's different from an aldehyde as it doesn't contain a hydrogen atom. It also contains highly electronegative atoms like oxygen, which result in a high boiling point and water solubility.
Ketone Levels and What They Mean
If you have diabetes, you will want to understand the difference between trace, moderate, and high ketone levels and what to do in each circumstance. Ketones typically develop when blood glucose levels are high.
When to Test for Ketones
Clinical recommendations are to test for ketones when blood sugars are elevated or when someone with diabetes is ill. These are provided so that early intervention can reduce the risk of DKA. 4
How to Test Ketones
Ketones can be tested at home via urine using a urine ketone strip or a blood ketone meter. To use a urine ketone strip, the strip is dipped into your urine sample and the color change is compared to a provided color array.
What to Do If Your Ketone Levels Are Off
If you have diabetes or are the caretaker of someone who does, you should always have a care plan for managing ketones. If you do not have one, make sure you call your diabetes team to create one.
Summary
Ketones develop when the body cannot use sugar for fuel and starts to utilize fat instead. In people with diabetes, excessive ketone production can result in a dangerous condition called diabetic ketoacidosis. This is more common in people with type 1 diabetes but can occur in anyone with diabetes.
A Word From Verywell
Detecting and treating ketones early can prevent an emergency. Understanding when, how, and what to do with your ketone information is important. Make sure you have supplies to check for ketones, plenty of sugar-free beverages, and access to your ketone management plan.
Overview
Structure and properties
The ketone carbon is often described as "sp hybridized", a description that includes both their electronic and molecular structure. Ketones are trigonal planar around the ketonic carbon, with C−C−O and C−C−C bond angles of approximately 120°. Ketones differ from aldehydes in that the carbonyl group (CO) is bonded to two carbons within a carbon skeleton. In aldehydes, the carb…
Nomenclature and etymology
The word ketone is derived from Aketon, an old German word for 'acetone'.
According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix -ane of the parent alkane to -anone. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered re…
Characterization
An aldehyde differs from a ketone in that it has a hydrogen atom attached to its carbonyl group, making aldehydes easier to oxidize. Ketones do not have a hydrogen atom bonded to the carbonyl group, and are therefore more resistant to oxidation. They are oxidized only by powerful oxidizing agents which have the ability to cleave carbon–carbon bonds.
Ketones and aldehydes absorb strongly in the infra-red spectrum near 1700 cm . The exact positio…
Synthesis
Many methods exist for the preparation of ketones in industrial scale and academic laboratories. Ketones are also produced in various ways by organisms; see the section on biochemistry below.
In industry, the most important method probably involves oxidation of hydrocarbons, often with air. For example, a billion kilograms of cyclohexanone are produced annually by aerobic oxidation of cyclohexane. Acetone is prepared by air-oxidation of cumene.
Reactions
Ketones engage in many organic reactions. The most important reactions follow from the susceptibility of the carbonyl carbon toward nucleophilic addition and the tendency for the enolates to add to electrophiles. Nucleophilic additions include in approximate order of their generality:
• With water (hydration) gives geminal diols, which are usually not formed in ap…
Biochemistry
Ketones are pervasive in nature. The formation of organic compounds in photosynthesis occurs via the ketone ribulose-1,5-bisphosphate. Many sugars are ketones, known collectively as ketoses. The best known ketose is fructose; it exists as a cyclic hemiketal, which masks the ketone functional group. Fatty acid synthesis proceeds via ketones. Acetoacetate is an intermediate in the Krebs cycle which releases energy from sugars and carbohydrates.
Applications
Ketones are produced on massive scales in industry as solvents, polymer precursors, and pharmaceuticals. In terms of scale, the most important ketones are acetone, methylethyl ketone, and cyclohexanone. They are also common in biochemistry, but less so than in organic chemistry in general. The combustion of hydrocarbons is an uncontrolled oxidation process that gives ketones as well as many other types of compounds.