What is the noble gas electron configuration of Mg2+?
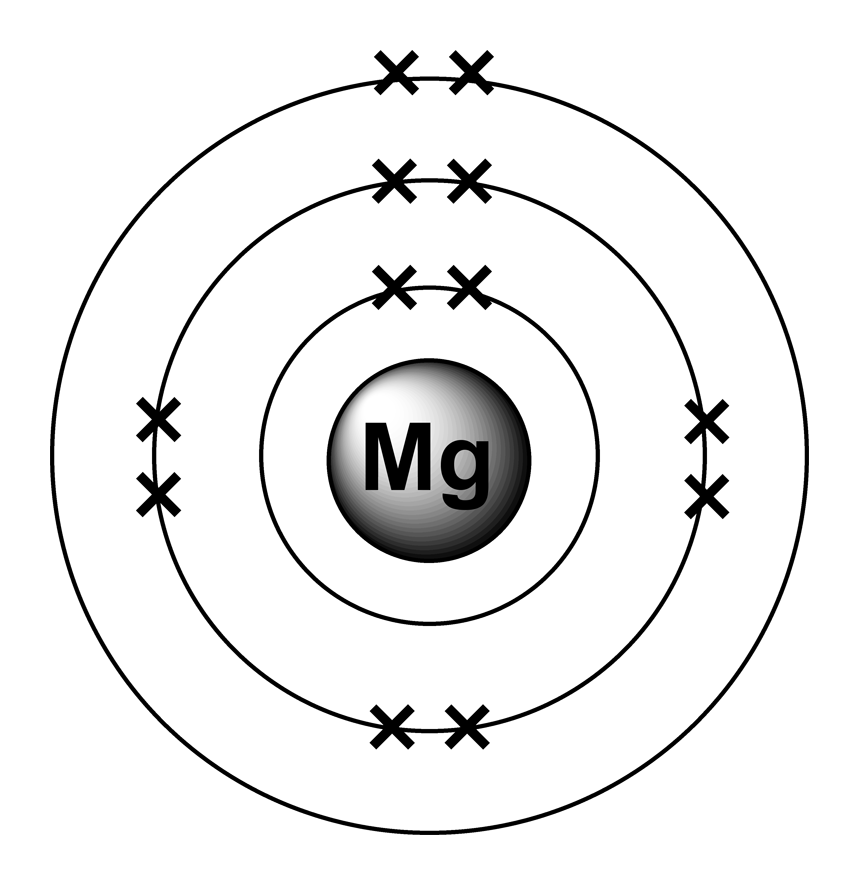
26/03/2021 · Mg atom consists of 12 electrons with the electronic configuration as 2, 8, 2. Mg2+ means 2 positive charge, which means 2 electrons are lost, thus no. of electrons remaining in the atom is 12– 2 =10 .
What is the electron configuration for magnesium in 1s 2s 2p?
24/06/2021 · What is the electron configuration of Mg 2? The electron configuration for magnesium is 1s 2 2s 2 2p 6 3s 2.
What does 2+ mean in Mg2+?
27/11/2020 · The number of electrons for the Mg atom are 12 electrons. The electron configuration of magnesium is, Mg (Z= 12) = 1s 2 2s 2 2p 6 3s 2. The first two electrons is placed in the 1s orbital. The 1s orbital can accommodate two electrons. The next 2 electrons for magnesium go in the 2s orbital. The next six electrons will go in the 2p orbital.
What is the ionic compound with the formula Mg^2+?
Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2. Video: Magnesium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.
How do you write electronic configuration?
The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital. For example: Looking at the periodic table, you can see that Oxygen has 8 electrons.
What is the electronic configuration of Magnesium Magnesium has 12 electrons?
Magnesium, atomic number 12, has the electron configuration [Ne] 3s ^2.
Does magnesium have 12 electrons?
So… for the element of MAGNESIUM, you already know that the atomic number tells you the number of electrons. That means there are 12 electrons in a magnesium atom.
What is the electron distribution in the atom with 12 electrons?
As we know that the atomic number of Magnesium is 12, so total 12 electrons will be distributed in a Magnesium atom .
What is the electron configuration of magnesium?
The electron configuration of magnesium is, Mg (Z= 12) = 1s 2 2s 2 2p 6 3s 2. The first two electrons is placed in the 1s orbital. The 1s orbital can accommodate two electrons. The next 2 electrons for magnesium go in the 2s orbital.
How many electrons can a p orbital hold?
The p orbital can hold up to six electrons. We’ll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore, the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
How many electrons are in a magnesium atom?
In order to write the Mg electron configuration we first need to know the number of electrons for the Mg atom (there are 12 electrons). When we write the configuration we'll put all 12 electrons in orbitals around the nucleus of the Magnesium atom. In writing the electron configuration for Magnesium the first two electrons will go in the 1s orbital.
How many electrons are in the 2p orbital?
The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s 2 2s 2 2p 6 3s 2.
Why is configuration notation important?
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Properties of Magnesium (Mg)
When binding with other elements (usually non-metals), Group IIa elements, i.e., alkaline earth metals such as Magnesium, are characterized by their tendency to lose or gain two electrons to form a type of bond called ‘Ionic Bond.
Order of Electron Arrangement
n, The diagram below shows us how electrons are arranged around each shell or orbital and how many each shell can ca to understand this shift in quantum energy level or loss of electrons. The Aufbau Principle guides this principle of arrangement