What is the difference between bromine and chlorine?
Bromine Vs Chlorine. Bromine and chlorine will sanitize and oxidize pool or spa water, but bromine works better at higher temperatures and is softer on the skin, whereas chlorine is cheaper, works for longer, and doesn’t break down as quickly in ultraviolet light, especially when coupled with cyanuric acid.
Why is bromination of alkanes more selective than chlorine?
Bromination of alkanes occur by a alike mechanism, but is slower and additional selective because a bromine particle is a less hasty hydrogen concept agentthan a chlorine particle, as reflect by the superior bond energy of H-Cl than H-Br. Which is more reactive among chlorine and fluorine?
What is the difference between endothermic bromination and exothermic chlorination?
The transition states for the endothermic bromination have a larger energy difference than those for exothermic chlorination, even though the energy difference of the products is the same in both reactants.
Is chlorine or bromine sanitizer better?
Bromine is widely known for having an imperceptible odor. Pool water treated with bromine has fewer reports of bad eye or skin reactions. As bromine has no bleach, water has less effect on clothes. The more stable the sanitizer, the safer it is to use. Is chlorine or bromine more stable? Let’s find out. Chlorine is safe.
See more
Which is faster bromination or chlorination?
Chlorination is faster than bromination because chlorine is more reactive.
Is bromine the same as chlorine for a pool?
Bromine and chlorine will sanitize and oxidize pool or spa water, but bromine works better at higher temperatures and is softer on the skin, whereas chlorine is cheaper, works for longer, and doesn't break down as quickly in ultraviolet light, especially when coupled with cyanuric acid.
Why is chlorination more reactive than bromination?
Although the bromine nucleus is more positively charged than the chlorine nucleus, the increase in the radius and the extra shielding in the bromine atom outweigh this factor, which means that an electron is more easily attracted into the outer shell of a chlorine atom than that of a bromine atom, so chlorine is more ...
Is chlorination more selective than bromination?
0:005:04Selectivity in radical chlorination vs bromination of alkanes - YouTubeYouTubeStart of suggested clipEnd of suggested clipOkay so this video is about the radical chlorination vs. The vertical bromination of alkanes. AndMoreOkay so this video is about the radical chlorination vs. The vertical bromination of alkanes. And the curious observation that chlorination of an alkane is normally if there's selectivity possible
Which is better bromine or chlorine?
Stability. While chlorine may work more quickly, bromine is more stable than chlorine, especially in warm water. Chlorine: Dissipates more quickly than bromine, and therefore needs to be replaced more often. Bromine: Kills bacteria in your spa for a longer period of time than chlorine.
Can I switch from bromine to chlorine?
0:151:07How to convert my swimming pool from bromine to chlorine? - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe the plumbing system all the lines have to have the water removed. In theory if you could justMoreThe the plumbing system all the lines have to have the water removed. In theory if you could just chemically. Remove all of the the bromine from the system you could just begin to use chlorine.
Is chlorine more stable than bromine?
Bromin is more stable than the Chlorine. Br is more stable than Cl because the more stable the reactant is, the less reactive it will be.
Why does chlorine displace bromine?
When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Because chlorine is more reactive than bromine, it displaces bromine from sodium bromide.
Why does chlorine have a greater electron affinity than bromine?
On moving from chlorine to bromine to iodine, the electron affinity decreases (becomes less negative). This is because the increase in atomic size decrease the effective nuclear charge. Hence, the additional electron feels less attraction by the large atom.
Why chlorination is more reactive and less selective?
Chlorination is less selective than bromination because chlorination has smaller differences in activation energy for attack at 1°, 2°, and 3° positions. Consider the halogenation of propane at the 1° and 2° positions. Formation of the different halopropanes occurs during the chain propagation steps.
Which catalyst is used in chlorination?
C1-symmetric amino sulfoximine–copper complex 23 has been used as chiral catalyst in chlorination reactions of β-keto esters in the presence of NCS as a source of electrophilic chlorine (entry 6, Table 1).
Why is chlorine not selective?
Since chlorine is a rather reactive reagent, it shows relative low selectivity, that means Cl2 does not discriminate greatly among the different types of hydrogens atoms (primary, secondary or tertiary) in an alkane.
What is bromination reaction?
Similarly, what is bromination reaction? Bromination: Any reaction or process in which bromine (and no other elements) are introduced into a molecule. Bromination of an alkene by electrophilic addition of Br2. Bromination of a benzene ring by electrophilic aromatic substitution. Bromination of a benzylic position by a free radical substitution reaction.
Is bromination endothermic or exothermic?
In chlorination, the reaction is exothermic, and the transition state resembles the reactants. In bromination, the reaction is endothermic, and the transition state resembles the products. According to Hammond's postulate we say that this transition state is “late”.
Is chlorination faster than bromination?
Comparison of rate of reaction in bromination and chlorination. I did research, about this one but the data and other things prove chlorination is faster than bromination, because chlorine is more reactive. But, some data says reaction of chlorine is more exothermic then bromine, that is the reason.
What is the difference between chlorine and bromine?
The big difference between chlorine and bromine here is going to be how they act in warm water. If you’re a spa owner, make sure you take notes here. Bromine is a lot more stable than chlorine (obviously, because of how it functions more slowly) but especially so in warm water.
Why is bromine not used as much as chlorine?
The main reason that bromine isn’t used nearly as much as chlorine is the cost. Depending on where you buy, it can cost anywhere from a few bucks more to (possibly) double the price of chlorine.
Which is better, chlorine or bromine?
Bromine and chlorine will sanitize and oxidize pool or spa water, but bromine works better at higher temperatures and is softer on the skin, whereas chlorine is cheaper, works for longer, and doesn’t break down as quickly in ultraviolet light, especially when coupled with cyanuric acid.
How does bromine work?
Bromine, on the other hand, works by a process called ionization. It’s the same basic idea: bromine combines with bacteria but actually forces apart the chemical bonds of its adversary. Bromine also has a lower pH than chlorine, so balancing your water chemistry is going to be a breeze using this sanitizing method.
What is chlorine used for in a pool?
Whether you’re a veteran pool/spa owner or a newbie to all things pool-related, you’ve probably heard of chlorine and its use as a water sanitizer. But have you heard of bromine?
Is bromine or chlorine better for pool?
Chlorine is a fast-acting chemical. It rips through the gunk in your pool very quickly and then is done. Bromine, although highly-reactive, is a little bit more laid back than chlorine. It works more slowly but works longer than chlorine.
Do you need to add bromine to a pool?
Although it looks like you need to use more bromine than chlorine to get the same outcome, the reality is that you don’t have to add bromine to your pool or spa as often. So… it evens out.
Which is better, bromine or chlorine?
Bromine works better at higher temperatures than chlorine. Above 75°F, bromine remains stable, whereas chlorine is more effective in temperatures as low as 65°F. This makes bromine a better choice for hot tubs and spas, and an unheated pool will be better served by the use of chlorine.
How long does bromine last?
One application of bromine can last up to a week, while chlorine needs to be added every other day or so.
How to apply chlorine to water?
Chlorine can be applied in three different ways: granules, liquid, or tablets. The granules or liquid can be added directly to the water without any specialized equipment, while the tablets are placed in a floating dispenser to maintain adequate levels of chlorine in the water.
How does chlorine work in a pool?
When chlorine is added to the pool water in a shock treatment, it quickly combines with the bacteria and kills it and is then largely burned up and eliminated by the filters.
How much does bromine cost in a pool?
If you use tablets, you must spend around $20 for a floating feeder to dispense the chlorine. The same pool maintained with bromine will cost about $1,000 a year for pool chemicals. In addition, you may have to spend from $150 to $400 on the installation of a bromine feeder.
What is chlorine in swimming pools?
See full cheat sheet. 1 Chlorine: A chemical added to the water in a swimming pool to kill bacteria and microorganisms that can make people sick. We are asking a few questions so that we can get you better cost estimates.
Is bromine better for an indoor pool?
This makes bromine a better choice for an indoor pool or spa.
What is free radical halogenation?
It doesn't really sound like you have a specific question. Free radical halogenation is about the simplest concept you will tackle in O-Chem. It sounds like you are in O-Chem 1, but it would seem to me that you would be tackling conjugated systems at this point in the semester.#N#As far as what is the difference between CL2 and Br2, in general if you can use chlorine you can also use bromine. There are some exceptions, but I'm sure your professor/book will cover it when the time is right.
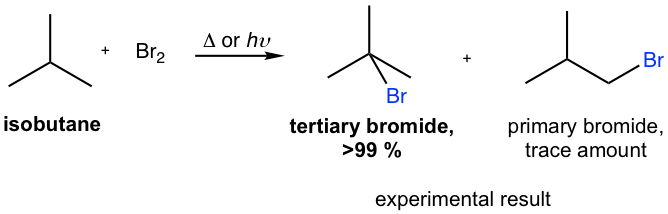
Is bromination more selective than chlorination?
In addition to what everyone else said, bromination is more selective than chlorination . So that means you'll get about a 60:40 ratio between chlorines added to a secondary carbon to chlorines added to a primary carbon. On the other hand, bromination would give about a rough 97:3 estimate between bromines added to a secondary carbon to bromines added to a primary carbon. Either way, when in light, these halogens add to carbons via the free radical mechansim. Your textbook should give a good outline of the details of the mechanism.
What is the difference between chlorine and bromine?
One major difference between chlorine and bromine is you would not use the latter to sanitize drinking or cooking water. There are plenty of uses for bromine. It’s an oxidizer, sanitizer, and algaecide. Many spa, pool, and hot tub owners use bromine instead of chlorine.
How to dissolve chlorine granules?
Pour the mixture into a second container and add water. Get the granules to dissolve by stirring the mixture.
Why do we use chlorine in water?
When extracted, we use chlorine to clean drinking water. Chlorine is a killer of pathogens by destroying chemical bonds in contaminants. Disinfectants use chlorine compounds in a process that exchanges compounds like atoms. It attacks harmful enzymes in bacteria and viruses.
What happens when chlorine confronts enzymes?
It attacks harmful enzymes in bacteria and viruses. When chlorine confronts enzymes, a chemical reaction occurs. Chlorine replaces hydrogen atoms. The molecule either falls apart or changes shape. Unable to function properly, the failure of enzymes leads to cells or bacteria dying.
Is bromine more stable than chlorine?
Bromine remains stable at higher temperatures than chlorine will . The chemical composition of bromine reacts differently than chlorine and ends up doing a more efficient cleaning of water in hot temperatures. This is why there will be fewer applications of the solution than chlorine.
Does chlorine bleach water?
Chlorine contains bleach. It has a very distinct smell and can be irritable to the skin. Bromine is widely known for having an imperceptible odor. Pool water treated with bromine has fewer reports of bad eye or skin reactions. As bromine has no bleach, water has less effect on clothes.
Does bromine neutralize chlorine?
Bromine neutralizes harmful pollutants in water. It does so continuously and for significant amounts of time. This is a great advantage over chlorine as the bromine application isn’t needed as much to disinfect as chlorine.