Distinguish Between Binary Acids and Oxyacids
- Definition. Binary Acids: A binary acid is a binary compound where one element is hydrogen, and the other is a nonmetal.
- Components. Binary Acids: Binary acids essentially contain a hydrogen atom bonded to another element. ...
- Oxygen. Binary Acids: Binary acids do not contain oxygen. ...
- Strength. ...
- General Formula. ...
- Conclusion. ...
How is a binary acid different from a ternary acid?
The key difference between binary and ternary acids is that binary acids are chemical compounds that are composed of hydrogen as an essential component that is bonded to a nonmetal whereas ternary acids are acid compounds containing hydrogen and oxygen atoms bonded to another element, most of the times, a nonmetal. 1. Overview and Key Difference 2.
Is hydrofluoric acid a binary acid or oxyacid?
binary acid. an acid that does not contain oxygen, such as hydrofluoric acid. oxyacid. an acid that is a compound of hydrogen, oxygen, and a third element, usually a nonmetal. arrhenius acid. a substance that increases the concentration of hydronium ions in aqueous solution. arrhenius base.
What are the rules for naming binary acid?
Summary
- Acids are molecular compounds that release hydrogen ions.
- A binary acid consists of hydrogen and one other element.
- Oxyacids contain hydrogen, oxygen, and one other element.
- The name of the acid is based on the anion attached to the hydrogen.
What two things does a binary acid contain?
Binary acids are certain molecular compounds in which hydrogen is combined with a second nonmetallic element; these acids include HF, HCl, HBr, and HI. HCl, HBr, and HI are all strong acids, whereas HF is a weak acid. The acid strength increases as the experimental pKa values decrease in the following order:
What is the difference between a binary acid and an oxyacid quizlet?
A binary acid contains hydrogen and one other element, whereas an oxyacid contains a hydrogen atom, one or more oxygen atoms and one other element.
Is an oxyacid a binary acid?
0:1913:11Binary Acid vs Oxyacid - YouTubeYouTubeStart of suggested clipEnd of suggested clipOr acids can be oxy assets. They're two different types both are considered to be assets. BecauseMoreOr acids can be oxy assets. They're two different types both are considered to be assets. Because they contain. Hydrogen. In the form of H+. So binary acids when we look at binary acids binary really
Why are binary acids stronger than oxyacids?
The key difference between binary acids and oxyacids is that oxyacids contain at least one oxygen atom in the molecule and binary acids do not contain oxygen. Binary acids have hydrogen and another non-metal element in the molecule.
What is oxyacid?
An oxyacid can be defined as an acid that contains an oxygen atom which is bonded to the hydrogen atom and also at least one of the other elements.
How do you identify oxyacids?
To name oxyacids, you must first be able to recognize them by the general formula HaXbOc, with X representing an element other than hydrogen or oxygen. It will also be useful for you to know the names of the polyatomic oxyanions, because many oxyacid names are derived from them.
How do you name binary acids and oxyacids?
1:277:54Naming Binary Acids and Oxyacids - YouTubeYouTubeStart of suggested clipEnd of suggested clipBut they're called oxyacids. Because they contain oxygens included with these hydrogen's and otherMoreBut they're called oxyacids. Because they contain oxygens included with these hydrogen's and other elements. All right so how do you name a binary acid all binary acids start with the prefix hydro.
Is h2so4 a binary or oxyacid?
Binary acids, such as hydrochloric acid, HCl(aq). Oxyacids, such as sulfuric acid, H2 SO4 , and nitric acid, HNO3 .
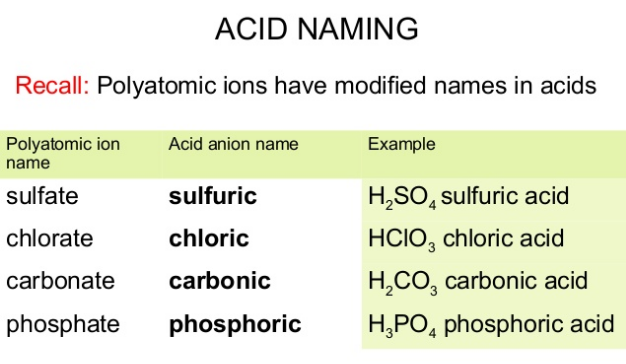
How are oxyacids named?
Rules for Naming Oxyacids (anion contains the element oxygen): Since all these acids have the same cation, H+, we don't need to name the cation. The acid name comes from the root name of the oxyanion name or the central element of the oxyanion. Suffixes are used based on the ending of the original name of the oxyanion.
What are the 3 types of acid?
Usually acids can be divided into three major types. First one is binary acid, second one is oxyacid, and the last one is carboxylic acid.
What are oxyacids and give examples?
An oxyacid dissociates in water to form the H+ cation and the anion of the acid. An oxyacid has the general structure X-O-H. Examples: Sulfuric acid (H2SO4), phosphoric acid (H3PO4), and nitric acid (HNO3) are all oxyacids.
Which one is an oxoacid?
Iodine.
What are the four types of oxyacids?
Chlorine forms four types of oxoacids. That is HOCl (hypochlorous acid), HOClO (chlorous acid), HOClO2(chloric acid) and lastly HOClO3 (perchloric acid).
What is the difference between oxyacids and binary acids?
The main difference between binary acids and oxyacids is that binary acids have the general formula H-X whereas oxyacids have the general formula H-O-X. Thus, the general formula of these acids helps ...
What are binary acids and oxyacids?
Binary acids and oxyacids are two types of acidic compounds. Binary acids are compounds that always contain a hydrogen atom bonded to a different element; hence they are also known as hydracids. Here, the hydrogen atom is bonded to a nonmetal such as a halogen, sulfur, etc. Oxyacids are compounds that essentially contain oxygen. The main difference between binary acids and oxyacids is that binary acids have the general formula H-X whereas oxyacids have the general formula H-O-X. Thus, the general formula of these acids helps to distinguish between binary acids and oxyacids.
What is binary acid?
Definition. Binary Acids: A binary acid is a binary compound where one element is hydrogen, and the other is a nonmetal. Oxyacids: An oxyacid is an acid that contains an oxygen atom bonded to a hydrogen atom and at least one other element.
What is an oxyacid?
What are Oxyacids. An oxyacid is an acid that contains an oxygen atom bonded to a hydrogen atom and at least one other element. The general structure of an oxyacid is H-O-X. A compound having this formula can dissociate in the aqueous medium in two different ways as given below. X−O−H ⇄ (X−O) − + H +.
What are some examples of binary acids?
Followings are some examples of binary acids. Diatomic binary acids – HCl, HI, etc. Halogen-containing binary acids – HF, HCl, HBr and HI.
What is the formula for oxyacids?
It is the general formula of these acids that helps to distinguish between binary acids and oxyacids; binary acids have the general formula H-X whereas oxyacids have the general formula H-O-X.
Is binary acid a diatomic element?
The nonmetal is a chemical element in the p block of the periodic table of elements. Binary acids are not always diatomic molecules; they just have two different elements bonded to each other. The general formal is H-X. Binary acids are capable of donating hydrogen atoms to the medium (H + ).
Answer
Hydrochloric acid (HCl) is a binary acid because it is a molecule with a bond between hydrogen and a nonmetal (Cl). Meanwhile. chloric acid (HClO 3 ) is a oxyacid because it is composed of hydrogen,oxygen, and a nonmetal (Cl). Hydroiodic acid (HI) is a binary acid because it is a molecule with a bond between a hydrogen and a nonmetal (I).
Video Transcript
The difference between a binary acid and an ox, ES said, is that a binary acid is a compound that's made up between ah, hydrogen atom and, well, just 10. Well, just a n where n can be any halogen or nonmetal, and an oxy acid is a compound made up of hydrogen oxygen and, well, say and again, where end can be any nonmetal. So let's do some examples.