Lewis structure of CS2 (or Carbon Disulfide) contains two double bonds between the Carbon (C) atom and each Sulfur (S) atom. The Carbon atom (C) is at the center and it is surrounded by 2 Sulfur atoms (S). The Carbon atom does not have a lone pair while both the Sulfur atoms have 2 lone pairs.
What is the molecular geometry of the CS2 molecule?
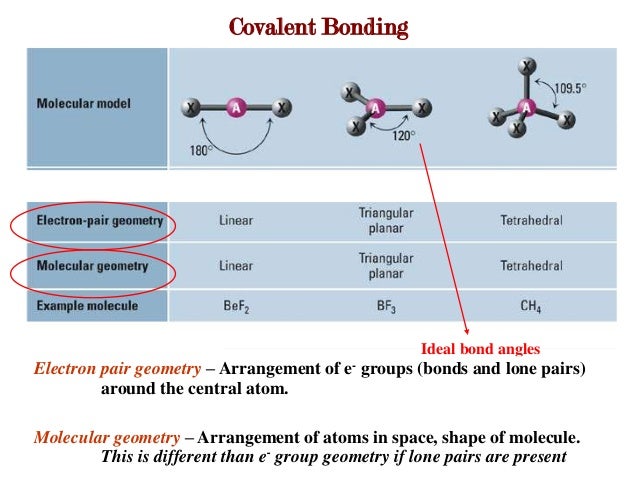
Molecular geometry. As the hybridization of CS2 is sp hybridization, the Carbon atom is in center bonding with two sulfur atoms forms the bond angle of 180 degrees, making the molecular geometry of CS2 molecule linear. The general formula for linear geometry is AX2, and thus CS2 shows linear geometry. Polarity. The polarity of the CS2 molecule depends on the geometry of the molecule.
How to write Lewis structure?
- Arrange the atoms to show specific connections. ...
- Determine the total number of valence electrons in the molecule or ion. ...
- Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. ...
- Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). ...
What is the molecular structure of CS2?
CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape. CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure. ぷっくりお花のキーホルダー ...
How do you know how many bonds are in a Lewis structure?
To know the no. of σ and π bonds the Lewis structure of the compound is to be drawn showing all single,double and triple bonds. In this structure Each single bond is 1σ bond
What is the structure of CS2 molecule?
The carbon atom will thus be sp hybridized. It will use one s and one p orbitals to form the hybrids, and the remaining p-orbitals to form pi bonds with the two sulfur atoms. The molecular geometry will thus be linear, the basic AX2 model.Nov 13, 2015
Is CS2 a structure?
CS2 is an organosulfur compound and a volatile liquid with chemical name Carbon Disulfide. It is also called Carbon bisulfide or disulfidocarbon or methanedithione.
What is the correct Lewis structure for co2?
1:474:08CO2 Lewis Structure - Carbon Dioxide - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe have a total of 16 valence electrons 2 4 6 8 10 12 14 16. So this is the Lewis structure of theMoreWe have a total of 16 valence electrons 2 4 6 8 10 12 14 16. So this is the Lewis structure of the carbon dioxide molecule.
What is the correct description for carbon disulfide?
Carbon Disulfide is a clear, colorless to light yellow liquid with an unpleasant, rotten egg odor as a reagent or commercial grade. Pure Carbon Disulfide has a sweet, pleasant odor. It is used to make rayon, cellophane and other chemicals, as a solvent, and a flotation agent. determine potentially hazardous exposures.
What do you mean by CS2?
Carbon disulfide, also spelled as carbon disulphide, is a neurotoxic colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent.
What is CS2 in chemistry?
Carbon disulfide or CS2 is one of the very common molecules we come across while studying chemistry. If you have ever come across the formation of carbon tetrachloride, a well-known reaction while studying chlorine, you have heard about CS2. Now let us learn about this sulfide in a detailed manner.
What is Lewis structure?
Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
How many valence electrons does sulfur have?
1. Carbon belongs to Group 4 of the periodic table. Therefore, the number of valence electrons in the Carbon atom =4. Sulfur (S) belonging to Group 6 has 6 valence electrons. CS2 has two S atoms, hence, the valence electrons in sulfur here are 6*2=12. Total valence electrons is CS2 = 12+4 = 16. 2.
How to draw the structure of a molecule?
The very first step towards drawing the structure of a molecule is to decipher the total number of valence electrons. (A valence electron is a name given to the outer shell electron of an atom that takes part in the creation of a chemical bond).
What is CS2 used for?
It can be used for the production of viscose rayon and cellophane.
What is the final step in Lewis diagram formation?
The final step of Lewis diagram formation is to verify whether all the atoms are in their lowest possible formal charge. (A formal charge, also abbreviated as FC is the charge that is assigned to an atom of a molecule when we assume that chemical bonds are always shared equally between atoms inside a molecule.)
Which atom exhibits sp hybridization?
If a central atom ( here C) has two valence electron density regions surrounding it, then it exhibits sp hybridization.
What is Lewis structure for CS2?
The Lewis structure for CS2 requires you have double bonds between the Carbon (C) and Sulfur atoms in order to fill the octet of Carbon.
How many valence electrons does CS2 have?
Transcript: Hi, this is Dr. B. Let's do the Lewis structure for CS2. On the periodic table, Carbon's in group 4, sometimes called 14, so it has 4 valence electrons. Sulfur in group 6 or 16, it has 6. We have two Sulfurs so let's multiply that by 2. Four plus 12: 16 valence electrons. Let's draw it.
What is CS2 in biology?
CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure.
How many orbitals does CS2 have?
Steric Number of CS2 is 2; thus its hybridization has two hybrid orbitals making it an sp hybridization.
How many sigma bonds are there in CS2?
Here in the CS2 molecule, the number of sigma bonds on the central atom is two , and there are no lone pairs on the central atom as its octet is complete by sharing the valence electrons. Steric Number of CS2 is 2; thus its hybridization has two hybrid orbitals making it an sp hybridization.
What is the name of the hybridization of carbon and sulfur?
This hybridization is known as sp hybridization . These two hybrid orbitals form sigma bonds with Carbon. Remaining eight valence electrons are taken up by the two unused orbitals of p. These electrons form the pi bonds with sulfur and are shown as the lone pairs on the sulfur atoms.
How to find the steric number of a molecule?
The formula to find the steric number for any molecule is: Steric Number (SN) = No of sigma bonds on the central atom +No of pi lone pairs on the central atom.
How to understand CS2 hybridization?
For understanding the hybridization of CS2 molecule, there are two easy methods. 1. To understand the bond formation and its type. It is essential to know the type of bonding in the molecule to understand its hybridization. In CS2 molecule, two double bonds are formed consisting of eight valence electrons.
What is the bond angle of CS2?
As the hybridization of CS2 is sp hybridization, the Carbon atom is in center bonding with two sulfur atoms forms the bond angle of 180 degrees, making the molecular geometry of CS2 molecule linear.
Lewis Structure of CS2
Hybridization of CS2
- Carbon disulfide has sp hybridization. Now, what is hybridization? Do you know that atoms use hybrid orbitals and not atomic orbitals to form chemical bonds in a molecule? This process of hybridization is one of the vital concepts of bonding and can be explained in two different methods- one with the help of theory and the other one depending on formulae. Let us first mak…
Molecular Geometry of CS2
- As discussed earlier, CS2 has a linear shape. Here, the bond angles form an angle of 180 degrees. To determine the molecular geometry of a molecule, we need to get familiar with a concept called VSEPR theory. Now, what is the VSEPR theory? VSEPR Theory is the short form for Valence Shell Electron Pair Repulsion Theory. It is based on the minimum repulsion concept where valence ele…
Polarity of CS2
- Before we discuss whether CS2 is polar or non-polar, let us just check a concise definition of polarity. Polarity is basically defined as the condition of having both positive and negative charges i.e. it denotes the distribution of electric charges across the atoms or molecules of a compound. A polar molecule will result due to unequal sharing of electrons whereas non-polar ones are neut…
Conclusion
- Understanding of any molecule requires a deep knowledge intake of the internal chemistry and bonding structure. Here, we have covered the Lewis Structure, Hybridization, Polarity, and Molecular Geometry of Carbon Disulfide in a well-explained and elaborative manner. This will help you grasp the basic concepts in a clear format. Apart from this, if you want to learn more, you ca…