| Property Name | Property Value | Reference |
|---|---|---|
| Formal Charge | 0 | Computed by PubChem |
| Complexity | 18.8 | Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07) |
| Isotope Atom Count | 0 | Computed by PubChem |
| Defined Atom Stereocenter Count | 0 | Computed by PubChem |
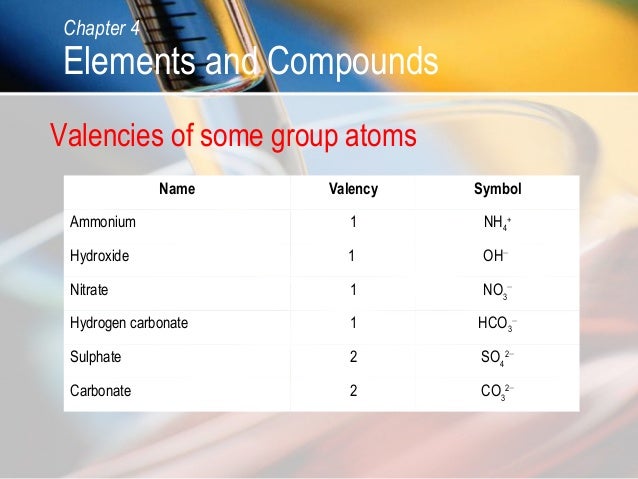
What is the charge of ammonium ion and carbonate ion?
Here, ammonium ion has +1 charge and carbonate ion has -2 charge. For them to form a salt which is electrically neutral in nature, every carbonate ion must be accompanied by 2 ammonium ions to nullify the Firstly a salt is formed by an anion (-vely charged species) and a cation (+vely charged species).
What is ammonium carbonate?
Ammonium carbonate appears as a colorless crystalline solid or a white powder with a strong odor of ammonia. Noncombustible. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in pharmaceuticals, in food processing.
What happens when ammonium carbonate is exposed to carbon dioxide?
It crystallizes in an ammonia solution exposed in a carbon dioxide-rich atmosphere. Ammonium carbonate slowly decomposes at standard temperature and pressure through two pathways. Thus any initially pure sample of ammonium carbonate will soon become a mixture including various byproducts.
Why do we need two ammonium cations to balance carbonate?
As you know, ionic compounds must be electrically neutral, meaning that the overall positive charge coming from the cation must be balanced by the overall negative charge coming from the anion. In this case, you need two ammonium cations to balance the 2− charge of the carbonate anion.
See more
What is the charge on ammonium in ammonium carbonate?
In this case, you know that you have ammonium, NH+4 , as the cation. The name of the anion follows the name of the cation. In this case, you know that you have the carbonate ion, CO2−3 , as the anion.
What is the formula of ammonium carbonate?
(NH4)2CO3Ammonium carbonate / Formula
Is ammonium carbonate cation or anion?
The chemical formula of ammonium carbonate: Here, the cation is ammonium ion and anion, carbonate ion.
What is the ammonium charge?
The formal charge on ammonium ion is +1.
How is ammonium carbonate ionic?
0:272:10Is (NH4)2CO3 (Ammonium carbonate) Ionic or Covalent?YouTubeStart of suggested clipEnd of suggested clipTogether the whole carbonate ion has a two minus ionic charge. So you can see we need two of theseMoreTogether the whole carbonate ion has a two minus ionic charge. So you can see we need two of these positive ammonium ions to balance the carbonate ion with the two minus charge.
Is ammonium carbonate neutral?
Ammonium carbonate is a very weakly acidic compound (based on its pKa).
Is ammonium carbonate ionic?
Ammonium carbonate actually contains both covalent and ionic bonds. The ionic bonds exist between the ammonium cations and carbonate anions, while the covalent bonds exist inside the polyatomic ions themselves (the N-H bonds for example).
How many ions are there for ammonium carbonate?
The chemical formula for ammonium carbonate is (NH4)2CO3 . The formula indicates that in one mole of ammonium carbonate, there are two moles of ammonium ions, NH4+ .
How do you balance ammonium carbonate?
0:011:41How to Balance (NH4)2CO3 = NH3 + CO2 + H2O - YouTubeYouTubeStart of suggested clipEnd of suggested clipMeans we have one times two two nitrogen atoms. Two times four eight hydrogen one carbon and threeMoreMeans we have one times two two nitrogen atoms. Two times four eight hydrogen one carbon and three oxygen atoms on the product side we have the one nitrogen.
Why ammonium has a positive charge?
Why ammonium ion contains a positive charge when hydrogen accepts lone pair of electrons from ammonia and forms coordinate bond . Why ammonium ion contains a positive charge when hydrogen accepts lone pair of electrons from ammonia and forms coordinate bond .
What is the formula and charge for ammonium ion?
Ammonium ionPubChem CID223Chemical SafetyLaboratory Chemical Safety Summary (LCSS) DatasheetMolecular FormulaH4N+Synonymsammonium Ammonium ion azanium Ammonium cation Ammonium(1+) More...Molecular Weight18.0393 more rows
What is the formal charge on NH4 positive?
Globally, therefore, the NH4 molecule is positively charged, i.e., it is a cation. This particular cation is called the ammonium ion. Notice that +1 and –1 formal charges in HN3 balance each other out. Overall, the molecule is electrostatically neutral.
What is the chemical formula for ammonium carbonate?
Ammonium carbonate is a salt with the chemical formula (NH 4) 2 CO 3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and was a predecessor to the more modern leavening agents baking soda and baking powder.
What is baker's ammonia made of?
Originally made from ground deer horn and called hartshorn, today it is called baker's ammonia. It is prepared by the sublimation of a mixture of ammonium sulfate and calcium carbonate and occurs as a white powder or a hard, white or translucent mass.
What is the purpose of sodium bicarbonate?
It acts as a heat activated leavening agent and breaks down into carbon dioxide (leavening), ammonia (which needs to dissipate) and water. It is sometimes combined with sodium bicarbonate to mimic as a double acting baking powder and to help mask any ammonia smell not baked out.
What is the charge of NH4+?
Firstly a salt is formed by an anion (-vely charged species) and a cation (+vely charged species). Here ammonium (NH4+) is the cation which carries +1 charge and carbonate ion (CO3 2-) carries -2 charge. When you do criss-cross you end up getting (NH4)2CO3 and that is ita chemical formula.
What is the chemical formula for crisscrossing salts?
When you do criss-cross you end up getting (NH4)2CO3 and that is ita chemical formula. Or logically if you think, salts are neutral species i.e they are electrically neutral having zero charge. Here, ammonium ion has +1 charge and carbonate ion has -2 charge.
What is the charge of carbonate?
This negative charge means that a single ion of carbonate has 2 more electrons than protons. Carbonate is a flexible polyatomic ion and is characterized by its tendency to form salt compounds with alkali and alkali earth metals. Carbonate compounds are the main component of several types of sedimentary rock, the most well known being limestone ...
What type of bond does carbonate form?
Carbonate ions are electrically negative, and so have a strong tendency to form ionic bonds with positively charged cations. The resulting substance is generally referred to as a carbonate salt. In general, carbonate ions form salts with group 1 and 2 alkali and alkaline earth metals.
What is the simplest oxocarbon ion?
Carbonate is the simplest kind of oxocarbon ion. it is made out of 3 oxygen atoms bonded to a central carbon atom and has a symmetrical trigonal planar geometry. Carbonate has a molar mass of about 60 g/mol. It is the conjugate base of carbonic acid (H 2 CO 3) and can be made via the dissociation of carbonic acid in a solvent.
How do ions form bonds?
Ions form bonds via the strong electrostatic attraction between positive and negative ions. In the case of sodium chloride (NaCl), a sodium cation will bond with a chlorine anion like so: An ionic bond between a sodium cation and a chloride anion. Credit: Rhannosh via WikiCommons CC BY-SA 3.0.
How do carbonate compounds contribute to the atmosphere?
They exude these carbonate compounds which in turn are converted into carbon dioxide and released into the atmosphere from Earth’s oceans. Carbonate systems in the oceans are one of the main natural producers of atmospheric carbon dioxide. Increasing ocean temperatures can result in the formation of more carbon dioxide from carbonate compounds dissolved in the ocean, leading to higher concentrations of carbon dioxide in the atmosphere.
What are carbonate compounds?
Carbonate compounds are the main component of several types of sedimentary rock, the most well known being limestone which is composed mainly of calcium carbonate (CaCO 3 ). Carbonate compounds also make up the shells of mollusks and coral, as well as in cleaning agents like sodium carbonate (Na 2 CO 3) and potassium carbonate (K 2 CO 3 ).
Why is carbonate important?
Carbonate is also an important biological substance. Most obviously, carbonate compounds are excreted by the human body to regulate internal pH levels. For example, when blood pH gets too low, meaning that the blood is acidic and has a high concentration of hydrogen ions, the body produces carbonate ions.
Ammonium Cation
A positively charged species that consists of a nitrogen atom attached to four hydrogen atoms is called an ammonium ion, or ammonium cation.
Ammonium Ion Formula
The representation indicating the constituent atoms (using symbols) of a compound, with their respective proportions, is called a chemical formula. The proportion of each element is indicated as its subscript, and overall charge is shown in superscript.
Ammonium Ion Lewis Structure
Lewis structures are the basic representation of the distribution of valence electrons, lone pairs, and charges on atoms, molecules, and ions. They also depict the bonding between the atoms in a molecule.