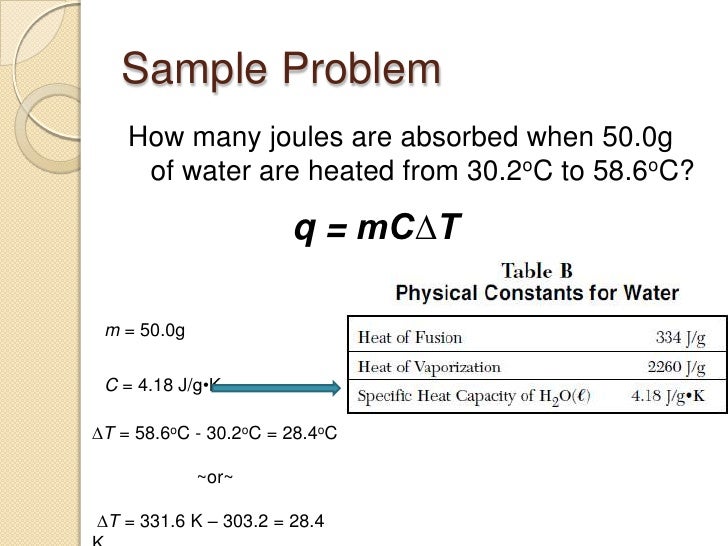
The quantitative relationship between heat transfer and temperature change contains all three factors: Q=mcΔT Q = mc Δ T , where Q is the symbol for heat transfer, m is the mass of the substance, and ΔT is the change in temperature. The symbol c stands for specific heat and depends on the material and phase.
What does C mean in Q MCT?
What does C mean in Q MCT? Specific heat capacity is the amount of heat that must be added or removed from a unit mass of a substance to change its temperature by one degree Celsius. The formula of specific heat is given by: Heat energy = Mass of substance × Specific heat × Change in temperature. Q = mc∆T.
What is the equation “q equals mc delta T”?
29/06/2020 · Click to see full answer. Similarly, it is asked, what is the equation Q MC T? The quantitative relationship between heat transfer and temperature change contains all three factors: Q=mcΔT Q = mc Δ T, where Q is the symbol for heat transfer, m is the mass of the substance, and ΔT is the change in temperature. The symbol c stands for specific heat and depends on the …
What is QQ=mcδt?
Q = mc∆T. Q = heat energy (Joules, J) m = mass of a substance (kg) c = specific heat (units J/kg∙K) ∆ is a symbol meaning "the change in". ∆T = change in temperature (Kelvins, K) Specific Heat Formula Questions: 1) The specific heat of gold is 129 J/kg∙K.
What are the units for Q in the equation Q=mcδt?
14/03/2017 · The C given in the problem is in units of kJ/C. You want your q to be in units of Joules or kJ. If you used the q=mC (delta T) with your given C, your q would be in units of (grams) (kJ). In the problem, you were given the heat capacity, not the specific heat capacity. Therefore, you don't need mass to calculate q.
What does C represent in Q MC T?
Q = mc∆T. Q = heat energy (Joules, J) m = mass of a substance (kg) c = specific heat (units J/kg∙K)
What is the C in Mcδt?
The amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcΔT, where m is the mass of the sample, c is the specific heat, and ΔT is the temperature change.
What is value of C in Mcdeltat?
The Specifice Heat Capacity of a material( c ), is the amount of heat energy that causes a change in temperature of 1K or 1°C per kg of that material.27-Mar-2015
What is CP in Q MC Delta T?
The one with just CdeltaT is used when you are just trying to find or are given the Heat Capacity. And the Cp is the heat capacity at constant pressure.
How do you find Q without C?
0:4213:08How to Calculate the Specific Heat Capacity of an Unknown Metal ...YouTubeStart of suggested clipEnd of suggested clipSo if I'm going to solve for C. Then what I'm going to do is take the original equation Q equals MCMoreSo if I'm going to solve for C. Then what I'm going to do is take the original equation Q equals MC delta T. And get C by itself by dividing away all the stuff that isn't C.
What is the C of water?
Water has a specific heat capacity of 4.186 J/g°C, meaning that it requires 4.186 J of energy (1 calorie) to heat a gram by one degree.18-May-2018
What does the C in mcAT stand for?
Specific heatm = Mass of a substance (kg) c = Specific heat (J/kg∙K)
How do I find my C on the mcAT?
5:157:01Using the formula q=mcΔT (Three examples) - YouTubeYouTubeStart of suggested clipEnd of suggested clipWell we can solve for C in Q equals MC delta T by dividing Q by M and delta T. So the amount ofMoreWell we can solve for C in Q equals MC delta T by dividing Q by M and delta T. So the amount of energy here is 968 joules the mass was 50 grams and the delta T is from 30 to 40.
How do you calculate joules?
In equation form: work (joules) = force (newtons) x distance (meters), where a joule is the unit of work, as defined in the following paragraph. In practical terms, even a small force can do a lot of work if it is exerted over a long distance.
What is Q in Q nC ∆ T?
Heat Capacity at Constant Volume Q = nCVΔT. For an ideal gas, applying the First Law of Thermodynamics tells us that heat is also equal to: Q = ΔEint + W, although W = 0 at constant volume.
What is Cp and CV?
CV and CP are two terms used in thermodynamics. CV is the specific heat at constant volume, and CP is the specific heat at constant pressure. Specific heat is the heat energy required to raise the temperature of a substance (per unit mass) by one degree Celsius.26-Jul-2017
What is Cp divided by CV?
The Cp/Cv ratio is also called the heat capacity ratio. ... (i.e.) Heat Capacity ratio = Cp/Cv = Heat capacity at constant pressure/ Heat capacity at constant volume.
What is the formula for Q?
To calculate the amount of heat released in a chemical reaction, use the equation Q = mc ΔT, where Q is the heat energy transferred (in joules), m is the mass of the liquid being heated (in kilograms), c is the specific heat capacity of the liquid (joule per kilogram degrees Celsius), and ΔT is the change in
What does Q mL mean in physics?
The specific latent heat (L) of a material… is a measure of the heat energy (Q) per mass (m) released or absorbed during a phase change. is defined through the formula Q = mL. is often just called the "latent heat" of the material. uses the SI unit joule per kilogram [J/kg].
Is Q delta H?
You can say that Q (Heat) is energy in transit. Enthalpy (Delta H), on the other hand, is the state of the system, the total heat content. They both can deal with heat (qp) (Q at constant pressure) = (Delta H) but both Heat and Enthalpy always refer to energy, not specifically Heat.
What does Q stand for in chemistry?
The reaction quotient Q is a measure of the relative amounts of products and reactants present in a reaction at a given time.
Is Delta T final minus initial?
The delta x means X final minus X initial and delta t is T final minus T initial. In other words we're finding the total displacement or the total change in distance over the total change in time.
What is specific heat example?
Definition: Specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. SYMBOL to denote it is c. Now best example to Specific heat is Water, for water specific heat is 1. real life example of specific heat: water takes more time to heat up and cool down.
What is CP and CV?
Cp is an amount of heat required to raise temperatire of an unit mass (1kg) by 1 degree Celsius when the system is at constant pressure. And Cv is an amount of heat required to raise the temperature of a unit mass by 1 degree Celsius when the volume of the system is constant.
Why does the equation not work if the substance changes states at that temperature?
This equation does not work if the substance changes states at that temperature because then energy is gained or lost by the change of state. An example of a state change is water going from solid to liquid form. ADVERTISEMENT.
What is the equation for specific heat?
Q=mcΔt is the equation for specific heat. Specific heat is the amount of heat per unit of mass that is needed to raise the temperature of the substance by 1 degree Celsius. Different substances have different specific heats since they require differing amounts of energy to change temperature by 1 degree Celsius.
What is Q in calorimetry?
It is actually Q= m c delta T. It comes in calorimetry. A hot substance which does not melt in water is dropped in water. The water temperature rises and the substance temperature reduces. The heat loss by the substance is equal to the heat gained by water. Q is the heat gained by water or lost by the substance.
What is heat energy?
Heat energy= (mass of substance) (specific heat ) (change in temperature) When heat energy is added to a substance,the temperature will change by certain amount, relationship between heat energy and temperature is different in different materials , specific heat describes how they relate.
What happens when Q is negative?
If q is negative, then energy has been released from the system to its surroundings, if q is positive, then energy has been drawn in from the surroundings to the system. Think of the system as a pot of water. When water is boiled, energy has to go into the system - in this case, energy in the form of heat.
What happens when water is boiled?
When water is boiled, energy has to go into the system - in this case, energy in the form of heat. The end result is that the average temperature of the water is greater than before (positive delta t). When the pot of boiling water cools, energy leaves the system, so the water is cooler than before (negative delta t).
What units do you use to mop up?
gives an answer that is always in energy units (ΔT has units of temperature) and if you need energy/mole, you can use mole/g * m (g ), and c (energy/mole.T). To mop up, don’t get tangled with units of T, by allowing a valu. Continue Reading.
Is the temperature of a solution or water an exothermic reaction?
The answer isnegative since the temperature has been raised which means it was an exothermic reaction ( therefore is -ve) You burn a fuel with a mass of water you know. Assuming all of the heat goes into the water . M is basically mass of a solution or water.
What is the enthalpy of combustion?
Secondly, the definition of the enthalpy of combustion is the enthalpy change when one mole of a substance is completely combusted in oxygen. You're trying to find the enthalpy change of combustion of propanol and you have used 0.5g (not 1 mole) so you divide the enthalpy change ( the energy released that you calculate using Q=mcT) ...