How do you find the bond order of F2?
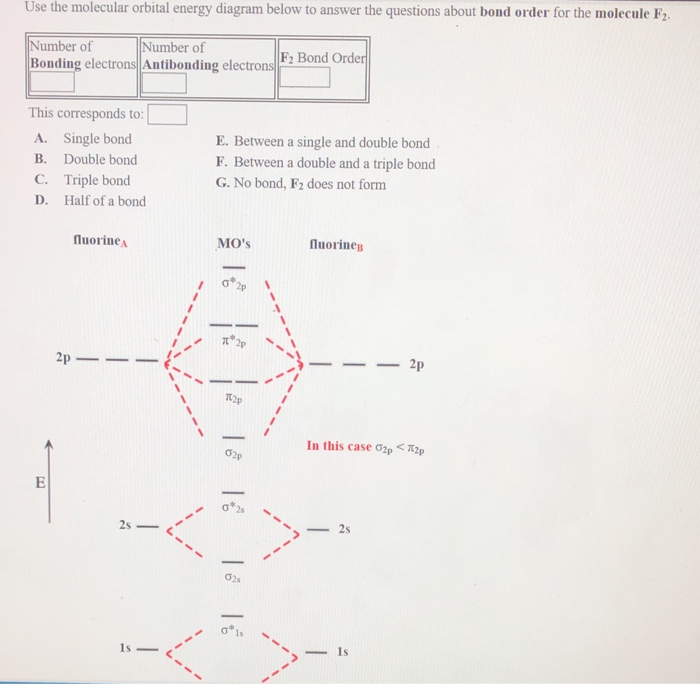
22/01/2022 · You can also find its bond order using advance Molecular orbital theory (MoT). Therefore Bond order of F2 is 1. The bond order is 1/2(no. of bonding electrons – no. of antibonding So in order of stability you have; F2+,F2.So,F2+>F2.
What type of bond is in F2 HF?
01/11/2021 · Answer- 1 is the bond order for an f2 molecule. We calculate the bond order with the help of molecular orbital theory or bond order theory. The theory and the explanation of how the f2 molecule has 1 as its bond order, which is detailed in the next sections of the article.
What is the structure of F2 molecule?
11/02/2022 · The bond order is 1/2(no. of bonding electrons – no. of antibonding So in order of stability you have; F2+,F2.So,F2+>F2. Similarly, what is the electron configuration of f2? Answer: F2 the following: (sigma 2s)^2.
What is the bond order of fluorine?
12/06/2021 · Originally Answered: What is the bond order of F2? In simple terms, since F has 7 valence electrons thus by sharing of electrons with another F it forms a bond to fullfil its octate. You can also find its bond order using advance Molecular orbital theory (MoT). Therefore Bond order of F2 is 1.Mar 26, 2021
What is the bond order of F2 and O2 2?
order 1F2 and O2^2 - have bond order 1 while N2, CO and NO^+ have bond order 3 .
How many bond does F2 have?
F2 lewis structure. There is only one single bond between two fluorine atoms and three lone pairs on each fluorine atoms. So, this lewis structure is a very simple.
Why is difluorine paramagnetic?
It's paramagnetic because it posses 2 unpaired electrons. For Difluorine, by counting the number bonding, 10 , and number of antibonding, 8 , give us the BO of 1. It is diamagnetic with no unpaired electrons. What is the formula for bond order?
What is the bond order of F2?
Therefore Bond order of F2 is 1. Click to see full answer. Moreover, what is the bond order for the f2 molecule? The bond order is 1/2 (no. of bonding electrons - no. of antibonding So in order of stability you have; F2+,F2.So,F2+>F2.
How many valence electrons does F2 have?
In simple terms, since F has 7 valence electrons thus by sharing of electrons with another F it forms a bond to fullfil its octate. You can also find its bond order using advance Molecular orbital theory (MoT). Therefore Bond order of F2 is 1. Click to see full answer.