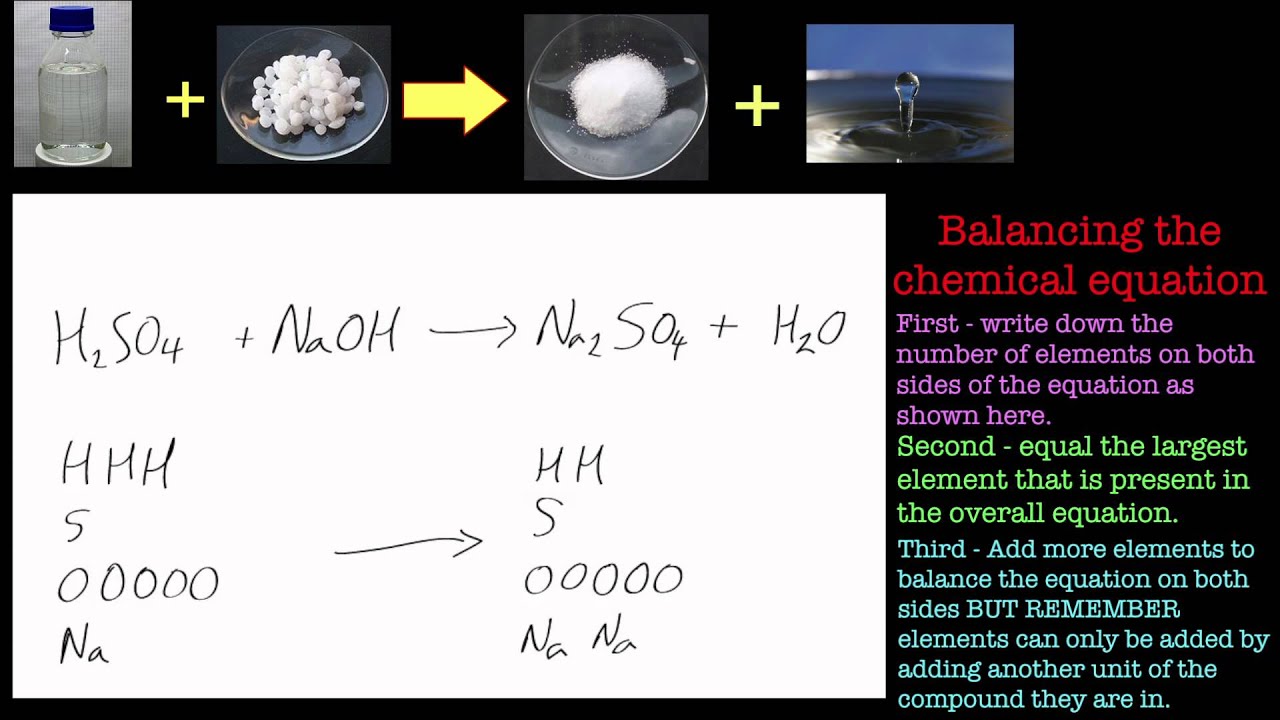
What is the balanced equation for the neutralization reaction of sulfuric acid with sodium hydroxide? 4) a) It is a good habit to start by inspecting the reaction by writing the balanced equation : 2 NaOH Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na⁺ and hydroxide anions OH⁻. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordi…Sodium hydroxide
What is the balanced equation for sulfuric acid and sodium hydroxide?
What is the balanced equation for the reaction of sulfuric acid and sodium hydroxide? H2SO4 + 2 NaOH => Na2SO4 + 2 H2O. What is the chemical equation for sulfuric acid and sodium hydroxide?
What happens when sodium hydroxide reacts with sulphuric acid?
When sodium hydroxide react with sulphuric acid they form Sodium sulphate and water. What neutralizes sulfuric acid? If you have a quantity of (concentrated) sulphuric acid, you can pour it into a solution of sodium hydroxide.
What is the balanced equation for the reaction between acetic acid and NaOH?
What is the balanced equation for the reaction between acetic acid and NaOH? Here's what I got. Acetic acid, CH3COOH, will react with sodium hydroxide, NaOH, to produce sodium acetate, CH3COONa, and water.
What is the reaction between H2SO4 and NaOH?
The reaction between sulfuric acid, H2SO4 , and the base sodium hydroxide, NaOH , is a neutralization reaction. Na2SO4 is the salt sodium sulfate. Secondly, what happens when you mix h2so4 and NaOH?
What is the word equation for the Neutralisation of sulfuric acid with sodium hydroxide?
H2SO4 + NaOH ----> Na2SO4 + H2O.Apr 4, 2020
Is NaOH H2SO4 balanced equation?
0:002:37How to Balance NaOH + H2SO4 = Na2SO4 + H2O - YouTubeYouTubeStart of suggested clipEnd of suggested clipTo balance this equation NaOH plus h2 so4 yields na2so4 plus h2o let's count up the atoms on eachMoreTo balance this equation NaOH plus h2 so4 yields na2so4 plus h2o let's count up the atoms on each side of the equation. For the sodium atom we have one of those oxygens.
What is the balanced equation for the neutralization reaction between H2SO4 and KOH in aqueous solution?
0:585:47write the balanced neutralization reaction between h2so4 and kohYouTubeStart of suggested clipEnd of suggested clipSo this is two oxygens. And I have two oxygens. So this here is now balanced. So this is this is theMoreSo this is two oxygens. And I have two oxygens. So this here is now balanced. So this is this is the balanced neutralization reaction between sulfuric acid and potassium hydroxide.
What type of reaction is H2SO4 2NaOH → Na2SO4 2H2O?
The given reaction is double displacement reaction.Jun 21, 2018
How do you balance H2SO4?
0:002:30How to Balance S + H2SO4 = H2O + SO2 (Sulfur + Sulfuric acid) - YouTubeYouTubeStart of suggested clipEnd of suggested clip1. Times 2 that gives us 2 so the sulfur is our balance let's update the oxygens. We have 1 plus 2More1. Times 2 that gives us 2 so the sulfur is our balance let's update the oxygens. We have 1 plus 2 times 2 that gives us 4 so now we have 5 oxygens everything's balanced except the oxygens.
What type of reaction is H2SO4 and NaOH?
The reaction between sulfuric acid, H2SO4 , and the base sodium hydroxide, NaOH , is a neutralization reaction.
What is the neutralization reaction of H2SO4 and KOH?
1 Expert Answer In this case our acid is sulfuric acid (H2SO4) and our base is potassium hydroxide (KOH). Now this is also a double replacement reaction, the H+ from the H2SO4 will attach to the OH- from the KOH to make H2O (water). Likewise, the K+ will stick to the SO42- to make Na2SO4 salt.Feb 12, 2020
Which of the following is the correct net ionic equation for the reaction of H2SO4 and KOH?
3:193:35How to Write the Net Ionic Equation for KOH + H2SO4 = K2SO4 + H2OYouTubeStart of suggested clipEnd of suggested clipSo that's the net ionic equation for Koh + h2 so4 potassium hydroxide plus water this is dr. B andMoreSo that's the net ionic equation for Koh + h2 so4 potassium hydroxide plus water this is dr. B and thanks for watching.
When H2SO4 is neutralized by NaOH in aqueous solution What is the net ionic equation?
I'm assuming that you're talking about the last reaction—H2SO4 (aq) + 2 NaOH (aq) → Na2SO4 (aq) + 2 H2O (ℓ). When we break this up into its ions, we get H2 (aq) + SO4(2−) (aq) + 2 Na+ (aq) + 2 OH− (aq) → 2 Na+ = SO4(2−) (aq) + 2 H2O(ℓ).
Is 2NaOH H2SO4 → Na2SO4 h2o a balanced equation?
A chemical reaction is said to be Balanced when the number of the atoms of elements in the reactants side is equal to that of the products side. Therefore, a balanced equation is given below. 2NaOH + 2H2SO4 ——– Na2SO4 + 2H2O.
How do we know when a chemical equation is balanced?
A chemical equation is balanced when the number of each kind of atom is the same on both sides of the reaction.Dec 10, 2016
What is the reaction to describe the neutralization of sulfuric acid?
0:135:32Sodium Hydroxide + Sulfuric Acid - Acid Base Neutralization ReactionYouTubeStart of suggested clipEnd of suggested clipWhen you mix these two together in equal amounts. They will neutralize each other such that you getMoreWhen you mix these two together in equal amounts. They will neutralize each other such that you get salt in water with a pH of around 7. Now let's talk about the products of this reaction.
What is the name of the acid that is neutralized by sodium hydroxide?
Neutralization of sulfuric acid (H2SO4) by sodium hydroxide (NaOH)proceeds in two steps as shown below.
What is the reaction of H2SO4 and NaOH?
Aqueous solution of sulfuric acid (H2SO4) reacts with aqueous solution of Sodium hydroxide (NaOH) and form aqueous solution of Sodium sulphate (Na2SO4) and liquid water.
What happens when sulfuric acid and zinc react?
When zinc and sulfuric acid react, the products are zinc sulfate and hydrogen gas. The acidity decreases as the reaction proceeds, assuming there is excess zinc available. The rate of reaction depends on the concentration of acid that remains and may also be limited by the surface area of the zinc (hydrogen gas bubbles may create a barrier which effectively reduces the contact between the acid and the metal) so as the sulfuric acid is depleted, the rate of reaction decreases, but it won’t completely stop until all of the acid reacts. Still there may be some acidity remaining for a long time.
What is the pH of H2SO4?
Even a dilute Solution of H2SO4 of concentration 0.01N, will have a pH=2!!.
Is NaOH a strong base?
Because NaOH (Sodium hydroxide) is a strong base and H2SO4 (sulfuric acid) is a strong acid.
Does adding alkali to a solution increase pH?
Further addition of the alkali will only increase the pH of the solution. O
Is sulfuric acid soluble in sodium carbonate?
When sodium carbonate and sulfuric acid react, again the chemicals are mutually soluble and again the reaction is not constrained to neutral pH. However one reaction product is carbon dioxide gas, which could interfere with mixing the chemicals to a degree. Nevertheless, the neutralization will be quite rapid if excess sodium carbonate is present.