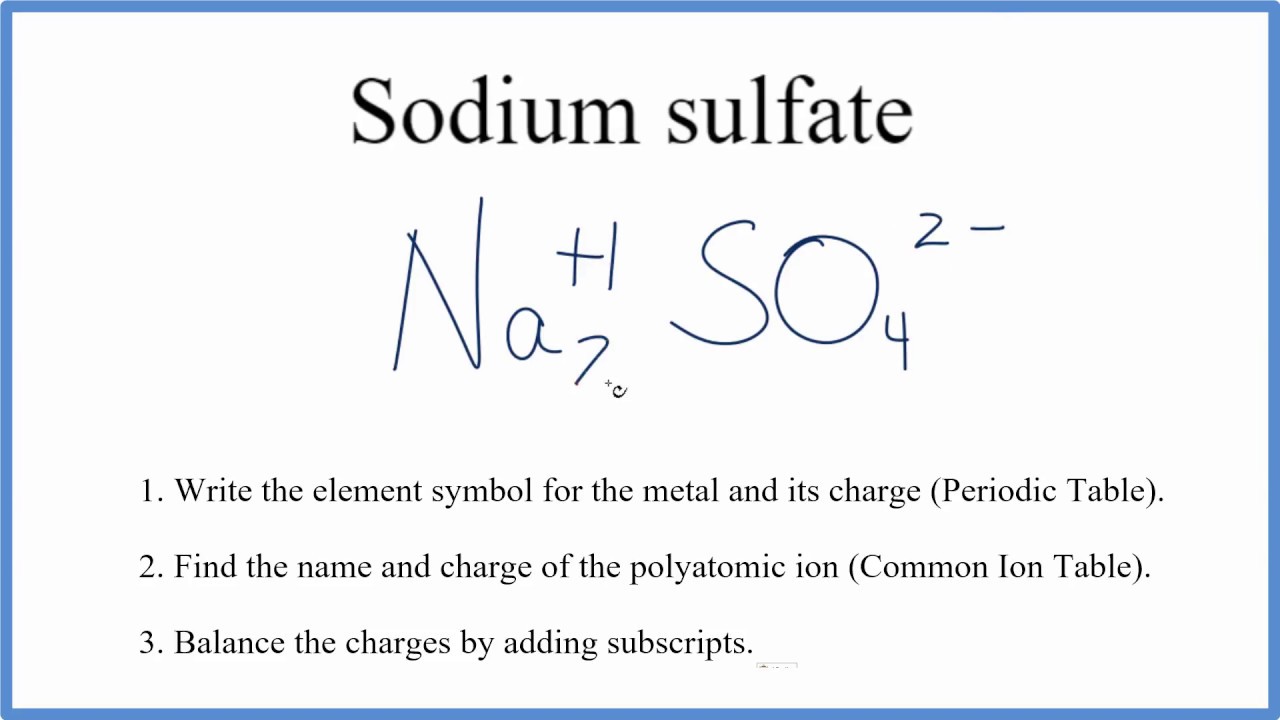
What is the charge on the polyatomic ion SO4?
For polyatomic ions containing oxygen and a chalcogen (S, Se, Te), the charge of the polyatomic ion is the same as the charge of the chalcogen: -2. For polyatomic ions containing oxygen and nitrogen, the charge is invariably -1. (Note that this is different th Good question, and one I have heard frequently in teaching Freshman Chemistry.
What does SO4 mean?
Sulphate Examples
- Magnesium Sulphate
- Copper Sulphate
- Gypsum
- Sodium Sulphate
- Iron (II) Sulphate
- Hydrogen Sulphate
- Calcium Sulphate
- Lead Sulphate
- Sodium Lauryl Sulphate
What is the correct charge for a chlorine ion?
What is the correct charge for a chlorine ion? Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Some elements, especially transition metals, can form ions of multiple charges. What is the name and charge of a chlorine ion?
What is the state of matter of SO4?
The notation for each state is as follows below: Gas = (g) Liquid = (l) Solid = (s) Aqueous = (aq) All the state of matter notations go at the end of each chemical next to the subscript. Examples: H 2 SO 4(aq) —> H 2(aq) + S (s) + O 2(aq) H 2(g) + O 2(g) —-> H 2 O (l) In the first equation, H 2 SO 4 is in an aqueous state. In the second equation, H 2 O is in a liquid state.
What is the formal charge of so4 2?
If the sulfur is assumed to have a single bond to each oxygen, the sulfur is assigned four electrons (half of the eight electrons in the four single bonds). Because a free sulfur atom has six valence electrons, the sulfur is this diagram is assigned a formal charge of +2.
Why is so4 negatively charged?
Oxygen needs 8 electrons around it (octet rule), so 6 come from its own valence electrons, another is shared by the sulfur and the final one is what makes up the negative charge in a S-O bond.
Is so4 negative 2?
Sulphate Structure [SO42-] As for the bonding, 2 of the oxygen atoms form S=O. bonds and the other two form S-O- bonds. The oxygen atoms are responsible for the negative charge (-2) of the anion because they are in a -2 state.
Is so4 positive or negative charge?
The molecular formula for sulfate is SO42-. Four bonds, two single and two double, are shared between the sulfur and oxygen atoms. The -2 you see on the sulfate ion reminds you that this molecule is charged. This negative charge comes from the oxygen atoms that surround the sulfur atom.16-Oct-2021
What is the charge of acetate?
negatively chargedThe molecular formula of acetate is C2H3O2-. Do you notice something unique with this formula? It contains a negative sign, indicating that this compound is negatively charged. Remember, the form of acetate that is negatively charged is called the acetate anion.15-Oct-2021
What charge is C2H3O2?
-13.1Computed PropertiesProperty NameProperty ValueReferenceFormal Charge-1Computed by PubChemComplexity25.5Computed by Cactvs 3.4.8.18 (PubChem release 2021.05.07)Isotope Atom Count0Computed by PubChemDefined Atom Stereocenter Count0Computed by PubChem12 more rows
How do you calculate charge on so4?
Charge on SulfateFormal Charge = 6 - 6 - (2/2) = -1.The oxygen atom labeled 4 will have the same formal charge.15-Oct-2021
What is the oxidation number of SO42?
The oxidation number of the sulfur atom in the SO42- ion must be +6, for example, because the sum of the oxidation numbers of the atoms in this ion must equal -2.
What is the charge of so4 3?
Notice that the aluminum ion has a charge of +3 and the sulfate ion has a charge of -2.
Is SO42 ionic or covalent?
The Lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms. This yields the expected total of 32 electrons. Since the sulfur atom started with six valence electrons, two of the S-O bonds are coordinate covalent.
Is NO3 positive or negative?
Nitrate, chemical formula NO3, has a chemical charge of -1. Ion nitrates have a negative one formal charge. You may be wondering why this is the case.15-Nov-2018
Is sulfate an anion?
The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−4. Salts, acid derivatives, and peroxides of sulfate are widely used in industry.02-Jul-2021
What is sulfate in chemistry?
Sulfate is considered a polyatomic anion. A polyatomic ion is a group of two or more atoms that behave as a single unit. Sulfate is an anion beca...
What is Sulfate made of?
Sulfate is a compound made of sulfur and oxygen. It is a polyatomic anion made of one sulfur atom and four oxygen atoms.
What is sulfate used for?
Organisms living near deep-sea thermal vents use sulfates as electron acceptors. Magnesium sulfate is commonly known as Epsom salts. These salts...
What is the formal charge of S in SO4?
The formal charge of sulfur in a sulfate compound is zero. This is due to its six different resonance structures.
How many electrons does sulfur have?
All these are related to its outer valence states electon as elemental sulfur contains 6 electrons in its valence shell ( 2 in s and 4 in p orbital) . For sulfide (- 2 ) you can add 2 more electrons to elemental sufur ( with 6 electrons; 2s and 4p ) ) to complete its octet ( stable configuration).
What is a sulphate ion?
Answered 3 years ago. In chemistry, a sulphate is a salt of sulphuric acid. The sulphate ion is a group of atoms with the formula SO4 and two negative charges. It consists of a central sulphur atom surrounded by four equivalent oxygen atoms.
How many atoms are in a sulphate?
One of them is the number of atoms in each element. In the following formula of sulphate, we have one atom of (s)sulphur and 4 atoms of (o). Therefore total atoms are 5.
What is the formal charge of an atom?
Formal charge of an atom = number of it’s valence electron before bonding - number of it’s valence electron after bonding. Ask for doubt. It has -2 sign due to formal negative charge on oxygen. Formal charge of an atom = number of it's valence electron before bonding - number of it's valence electron after bonding.
What happens when you work out the total charge of an anions?
If you work out the total charge on the anions, you will see the lower oxidation state of S on the sulfite anion gets compensated by a lower number of O atoms surrounding it. That makes sense: the higher the oxidation state on the central S atom, the less electrons it has because of the more O atoms around it.
How many resonance structures does a sulfite ion have?
In a more detailed explanation, the sulfite ion has 3 resonance structures. Each has a S=O bond (which has no charge), and 2 single S-O bonds which have a -1 charge each, as oxygen needs 2 bonds to be neutrally charged.
Can SO4 use 2p orbitals?
In the case of SO4 (-2) ion the S cannot use the 2p orbitals for pi bond formatio. Continue Reading. The reason for this lies in the difference in their structures. In CO3 (-2), the pi bonding electrons are in 2p orbitals for both carbon and oxygen.
How many electrons does sulfur donate to oxygen?
In fact, sulfur donates two electrons to the oxygen atoms. ^ The prefix "bi" in "bisulfate" comes from an outdated naming system and is based on the observation that there is twice as much sulfate (SO2−. 4) in sodium bisulfate (NaHSO4) and other bisulfates as in sodium sulfate (Na2SO4) and other sulfates.
How does sulfur dioxide affect the stratosphere?
Sulfate is also the major contributor to stratospheric aerosol formed by oxidation of sulfur dioxide injected into the stratosphere by impulsive volcanoes such as the 1991 eruption of Mount Pinatubo in the Philippines. This aerosol exerts a cooling effect on climate during its 1-2 year lifetime in the stratosphere.
What are sulfates in the atmosphere?
Sulfates occur as microscopic particles ( aerosols) resulting from fossil fuel and biomass combustion. They increase the acidity of the atmosphere and form acid rain. The anaerobic sulfate-reducing bacteria Desulfovibrio desulfuricans and D. vulgaris can remove the black sulfate crust that often tarnishes buildings.
How many resonances does sulfate have?
Two models of the sulfate ion. 1 with polar covalent bonds only; 2 with an ionic bond. Six resonances. The first description of the bonding in modern terms was by Gilbert Lewis in his groundbreaking paper of 1916 where he described the bonding in terms of electron octets around each atom, that is no double bonds and a formal charge ...
What is the structure of sulfate anion?
Structure. The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state.
What are sulfates used for?
Sulfates are widely used industrially. Major compounds include: 1 Gypsum, the natural mineral form of hydrated calcium sulfate, is used to produce plaster. About 100 million tonnes per year are used by the construction industry. 2 Copper sulfate, a common algaecide, the more stable form ( CuSO 4) is used for galvanic cells as electrolyte 3 Iron (II) sulfate, a common form of iron in mineral supplements for humans, animals, and soil for plants 4 Magnesium sulfate (commonly known as Epsom salts ), used in therapeutic baths 5 Lead (II) sulfate, produced on both plates during the discharge of a lead–acid battery 6 Sodium laureth sulfate, or SLES, a common detergent in shampoo formulations 7 Polyhalite, hydrated K 2 Ca 2 Mg-sulfate, used as fertiliser.
Is ionic sulfate soluble in water?
Properties. There are numerous examples of ionic sulfates, many of which are highly soluble in water. Exceptions include calcium sulfate, strontium sulfate, lead (II) sulfate, and barium sulfate, which are poorly soluble. Radium sulfate is the most insoluble sulfate known.