What is the balanced equation for bromine?
What Is The Balanced Equation Of Bromine + Sodium Iodide Yields Sodium Bromide + Iodine? The balanced equation of bromine plus sodium iodide yields sodium bromide plus iodine is;2NaI + Br2 ------> 2NaBr + I2Two moles of sodium iodide react with one mole of bromine to form two moles of sodium bromide and one mole of iodine.
What is the equation to remove bromine from water?
- Oxidizing Agents, Strong
- Halogenating Agents
- Water and Aqueous Solutions
What is bromine and why is it dangerous?
Bromine is dangerous because it is a corrosive liquid and a toxic, choking gas. Bromine is a chemical that is toxic to humans and has a wide range of side effects from contact or ingestion. The use of bromine in industrial applications and manufacturing has exposed some people to unusually high levels.
What is the equation of cyclohexane with bromine water?
If an aqueous solution of bromine is used ("bromine water"), you get a mixture of products. Identify X. The carbon-hydrogen bonds are only very The equation of cyclohexane and cyclohexene react with oxygen to produce carbon monoxide and water: C6H12 + 6O2 → 6CO + 6H2O.
What is the reaction between bromine and water?
Reactions: Bromine. Bromine reacts with water to produce hypobromite, OBr-. The pH of the solution determines the position of the equilibrium. Bromine is not reactive towards oxygen or nitrogen but it will react ozone at -78°C to form the unstable compound bromine(IV) oxide.
What is meant by bromine water?
Definition of bromine water : a solution of bromine in water especially : the red saturated solution.
How do you make bromine water?
How to Make Bromine Water. Pour the 1.1 g sodium bromine into the 10.7 sodium hypochlorite. Add the 7.6 ml hydrochloric acid and pour them into a glass bottle filled with 32 ml of water. Make sure the bottle has a secure, screw on top.Apr 24, 2017
What is bromine equation?
0:001:28Write the Formula for Bromine gas - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo if you have an equation for example hydrogen gas which is one of our diatomics that's alwaysMoreSo if you have an equation for example hydrogen gas which is one of our diatomics that's always going to be h2. That's a gas plus bromine gas bromine is going to always be br2 when it's bromine.
What is chlorine water formula?
Answer: Chlorine water is just a solution of water and chlorine. It is formed from the following reaction. Cl2 + H2O → HClO + HCL.
What is the bromine water test?
The bromine water test is a qualitative test, used to identify the alkene or alkane functional groups present in the compound. Alkene groups react with bromine water in the dark condition and undergo an addition reaction, to give a decolourized solution.
How do you make bromine water in a lab?
Dissolve the sodium bromine in the hydrochloric acid, mixing the compounds in a flask or beaker. Pour the mixture into the glass bottle. Add the bleach to the mixture in the bottle. Cap the bottle and swirl it gently to mix the ingredients.Apr 24, 2017
What concentration is bromine water?
Bromine Water, Saturated, approximately 3% (w/v), Ricca Chemical | Fisher Scientific.
What color is bromine water?
Bromine water is an orange solution of bromine. It becomes colourless when it is shaken with an alkene. Alkenes can decolourise bromine water, but alkanes cannot. The slideshow shows this process.
Why is bromine liquid?
Bromine, on the other hand, has a slightly higher molecular weight than fluorine and has stronger intermolecular interactions, thus it persists as a liquid at ambient temperature. Because iodine has a large molecular weight and strong Van Der Waals forces, it exists as a solid at normal temperature.
What is the formula for bromine Monochloride?
BrClBromine monochloride / Formula
Is bromine soluble in water?
Bromine is slightly soluble in water and highly soluble in many organic solvents, including carbon disulfide, carbon tetrachloride, acetic acid, and aliphatic alcohols.
What is the bromine formula?
Bromine is a diatomic halogen with the formula ({rm{B}}{{rm{r}}_2}).
Why is Bromine written as ({rm{B}}{{rm{r}}_2})?
Bromine is found in group (7;{rm{A}}) of the periodic table, which means it has seven valence electrons. To complete its octet, it only needs to ga...
Is ({rm{B}}{{rm{r}}_2}) Gas or liquid?
({rm{B}}{{rm{r}}_2}) is a reddish-brown coloured element, which is liquid at room temperature.
Is Bromine hazardous?
Bromine is corrosive to human tissue in a liquid state, and its vapours irritate the eyes and throat. Bromine vapours are very toxic with inhalatio...
Where is Bromine found in nature?
Bromine is found naturally in the Earth's crust and seawater in various chemical forms. It is present in the Earth's crust in compounds as soluble...
What is the difference between ({rm{Br}}) and ({rm{B}}{{rm{r}}_2})?
({rm{Br}}) is the chemical symbol of the bromine atom whereas ({rm{B}}{{rm{r}}_2}) is the molecular symbol of dibromine. As Bromine cannot occur in...
What is bromine water?
Bromine water is an oxidizing, intense yellow-to-red mixture containing diatomic bromine (Br 2) dissolved in water (H 2 O). It is often used as a reactive in chemical assays of recognition for substances which react with bromine in an aqueous environment with the halogenation mechanism, mainly unsaturated carbon compounds (carbon compounds with 1 or more double or triple bond (s)). The most common compounds that react well with bromine water are phenols, alkenes, enols, the acetyl group, aniline, and glucose. In addition, bromine water is commonly used to test for the presence of an alkene which contains a double covalent bond, reacting with the bromine water, changing its color from an intense yellow to a colorless solution. Bromine water is also commonly used to check for the presence of an aldehyde group in compounds. In this reaction as well the color of bromine water is changed to colorless from yellow (oxidation process).
Why is bromine water used in oxidation?
Bromine water is also commonly used to check for the presence of an aldehyde group in compounds. In this reaction as well the color of bromine water is changed to colorless from yellow (oxidation process).
What compounds react well with bromine?
The most common compounds that react well with bromine water are phenols, alkenes, enols, the acetyl group, aniline, and glucose. In addition, bromine water is commonly used to test for the presence of an alkene which contains a double covalent bond, reacting with the bromine water, changing its color from an intense yellow to a colorless solution. ...
What is the chemical formula for bromine water?
Bromine water, also called Bromide bromate solution or Bromine solution, is a brown-red coloured solution with the chemical formula B r 2. It is prepared by dissolving diatomic Bromine ( B r 2) in water ( H 2 O).
What is the density of bromine water?
The molecular weight of bromine water is 159.81, and the density is ( 1.307 g / m L). It has high oxidising properties.
What state does bromine water react with?
The reaction takes place at room temperature if the reactants are in the gaseous state (ethene). The colour of the bromine water solution is decolourised as it reacts with ethene.
How is bromine formed?
A bromine molecule or Dibromine is formed when two atoms of Bromine combine to attain stability.
How many electrons does dibromine have?
In the case of Dibromine, or ( B r 2) Both the Bromine atoms have 7 electrons in their outermost valence shell and need one more electron to attain stability.
What is the reaction of ketones with bromine water?
5. Ketones undergo an electrophilic alpha substitution reaction with bromine water and form a colourless solution of brominated compounds.
What is the formula for bromine trifluoride?
Bromine trifluoride is an interhalogen compound with the formula B r F 3.
How much bromine dissolves in water?
At room temperature, 3.41 grams (0.12 ounce) of bromine dissolve in 100 millilitres (0.1 quart) of water. Bromine water is the name for the solution. It is a good oxidising agent, similar to chlorine water, but it is more useful because it does not break down as quickly. It extracts free iodine and sulphur from iodide-containing liquids and hydrogen sulphide. Bromine water oxidises sulphurous acid to sulfuric acid. In the presence of sunlight, bromine water decomposes, releasing oxygen, as shown in the equation:
What is the name of the chemical that makes bromine?
The Bromine formula, also known as Dibromide formula or Brome formula. Diborane formula is the Br2 chemical name. It's the third-lightest halogen, and at room temperature, it's a seething red-brown liquid that quickly evaporates to form a similar-colored vapour. It has characteristics that are halfway between chlorine and iodine. Its name was derived from the Ancient Greek o ("stench"), referring to its strong and offensive scent. It was isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826).
What is bromine used for?
Bromine is employed in a variety of industries, including agriculture, dyes, pesticides, medicines, and chemical intermediates. Some usages are being phased out due to environmental concerns, while new ones are being discovered all the time. Flame retardants can be made from bromine compounds.
What metals does bromine react with?
Bromine reacts violently with alkali metals, as well as with phosphorus, arsenic, aluminium, and antimony, but less so with other metals. Bromine adds to unsaturated hydrocarbons and displaces hydrogen from saturated hydrocarbons, though not as easily as chlorine.
Where is bromine found?
The element is easily mined commercially from brine pools, which are predominantly found in the United States, Israel, and China. Bromine makes up around one-third of the mass of chlorine in the oceans.
Is bromine poisonous to humans?
Bromine, while liquid, is corrosive to human tissue, and its fumes irritate the eyes and throat. Bromine fumes are extremely poisonous when inhaled. Organic bromines, on the other hand, can harm organs like the liver, kidneys, lungs, and milt, as well as causing stomach and gastrointestinal problems.
Is bromine a weak bond?
however, a less effective oxidising agent, owing to the bromide ion's lesser hydration compared to the chloride ion. A metal-bromine bond is similarly weaker than a metal-chlorine link, and this difference is represented in bromine's chemical reactivity, which is intermediate between chlorine and iodine. Organic bromo compounds are similar to their chloro derivative counterparts but are usually denser, less volatile, less flammable, and less stable.
What is the formula for bromine?
Bromine formula has another name as Dibromine formula or Brome. It is a halogen and has an atomic symbol Br. In the year 1825-1826 scientists discovered the Dibromine. Those scientists were Antoine Ballard and Carl Jacob Lowing. Brome is a volatile type of liquid having a reddish-brown colour. It has a type of pungent odor but giving the suffocating vapors. It is soluble in water and is denser than water. Also.it is toxic and corrosive in nature as well. This topic will explain the Bromine Formula. Let us learn it here!
What is the boiling point of bromine?
The density of the Bromine is 3.1028 g per cubic cm. The boiling point of Bromine is 58.8 degrees C. Melting point of the Bromine is −7.2 degrees C. Also, inhaling 11-23 mg per cubic meter of this element will cause choking.
What is bromine used for?
One of the major uses of bromine is as a water purifier and as an alternative to the chlorine. Brominated compounds are useful for water treatment in swimming pools and hot tubs. It is also used to control algae and bacterial growth in industrial processes.
Where is bromine found?
The most visible form of bromine is from the soluble salts found in seawater, salt lakes, inland seas, and brine wells. The main areas of bromine production in the world are from salt brines found in the USA and China.
Is bromine a corrosive element?
Vapors of Bromine may cause acute and chronic poisoning. It is a corrosive element in nature. It may lead to death due to the circulatory collapse. Therefore the least oral lethal dose recommended for humans is 14 mg per kg weight.
Is bromine a halogen?
Bromine is like chlorine, fluorine, and iodine. It is one of the elements in the chemical group which is “halogens”. Bromine was discovered as a chemical element until 1826 when the French chemist Antoine Ballard isolated it from chlorine.
Is bromine a pesticide?
Bromine compounds are effective as pesticides. It is useful both as to soil fumigants in agriculture, and as a fumigant to prevent pests. In the past, bromine compounds were useful in leaded fuel, as a constituent of “anti-knock fluid”.
What is bromine oxidizing?
BROMINE is a powerful oxidizing agent . Reacts vigorously with reducing reagents. Can ignite a combustible material upon contact. If heated by itself or if mixed with water or steam, highly toxic and corrosive fumes are emitted. Reacts explosively with hydrogen, diethylzinc, dimethylformamide, ammonia, trimethylamine, nitromethane, metal azides ( silver or sodium azide ). Mixtures with lithium or sodium are shock-sensitive. Ignites on contact with germanium, trialkyl boranes, copper and alkali metal acetylides [Sax, 9th ed., 1996, p. 506]. Attacks most metals, including platinum and palladium [Hawley]. May react violently to form bromides upon contact with Mg, Sr, B, Al, Hg, Ti, Sn, Sb in powder or sheet form. Sodium, potassium, antimony and germanium ignite in bromine vapor and react explosively. Ignites on contact with germanium, trialkyl boranes, copper and alkali metal acetylides [Sax, 9th ed., 1996, p. 506]. Violent reaction with methanol, ethanol, aldehydes, ketones, carboxylic acids, diethyl ether, carbonyl compounds, tetrahydrofuran, acrylonitrile, ozone, phosphorus. Methyl acetylides or carbides ignite at room temperature on contact with bromine vapor. Explosive reaction with red phosphorus, metal azides, nitromethane, silane and its homologues [Bretherick, 5th ed., 1995, p. 109]. Reacts violently on contact with natural rubber [Pascal, 1960, vol. 16.1, 371].
What is the pesticide code for bromine?
For bromine (USEPA/OPP Pesticide Code: 008701) ACTIVE products with label matches. /SRP: Registered for use in the U.S. but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses./
What is the best solution for bromine fumes?
Ammonia soln should not be applied to liquid spills because of the high heat of reaction & nitrogen evolution. Anhydrous ammonia gas is useful for neutralization of bromine fumes.
How long does bromine react with hydroxyl radicals?
Vapor-phase bromine in the atmosphere (BR2) will react with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 8.6 hours. Bromine absorbs at wavelengths >290 nm and, therefore, may be susceptible to direct photolysis by sunlight.
How long does bromine exposure affect animals?
/LABORATORY ANIMALS: Acute Exposure/ / Bromine / exposure of 7 hours to 23 ppm provoked only slight irritation of respiratory tract and slight dyspnea in cats, rabbits and guinea pigs, while 180 ppm caused disturbances of function of CNS. Necropsy of guinea pigs and rabbits following 3 hour exposure at 300 ppm revealed edema of lung, a pseudomembranous deposit on trachea and bronchi, and hemorrhages of gastric mucosa. Foci of bronchopneumonia were found in animals that died several days after exposure and there was evidence of functional disturbances in CNS.
How is brine made?
Raw brine is warmed in a heat exchanger and caused to pass successively through two packed towers by gravity flow. In the upper (smaller) tower, it meets a recycle stream of gases from which chlorine and bromine are absorbed. Near the bottom of the lower tower, chlorine and steam are introduced; as they pass upward, the chlorine reacts with bromide in the brine, and a mixture of bromine vapor and chlorine (about 85:15 by weight) with steam is taken off the top. The water and most of the halogens are condensed, the liquid phases enters a gravity separator and the gas goes to the upper tower. From the separator, water containing dissolved halogens is sent to the lower (steaming-out) tower and the heavier bromine layer, containing some chlorine, flows to a fractionating column. The chlorine vapors join the stream to the upper tower while the liquid bromine, about 99 % pure, is drawn off either for direct use in the manufacture of bromine compounds or to be further purified for sale or for more exacting uses.
How does bromine enter the body?
Bromine vapors enter body by respiratory system, skin and digestive system. It has cumulative properties, being deposited in tissues as bromides.
How to make bromine brown?
The simplest way to make it is to put a small amount of bromine in the bottom of a jar and then add water, put a lid on it,and let it sit for a while. Some of the bromine will dissolve in the water and turn it brown.
What is bromine used for?
Bromine is used as a mild oxidizer and a reagent to add bromine to organic compounds.
What happens to bromine in the dark?
Nothing happens in the dark. In the light, the bromine substitutes into the hexane with the formation of hydrogen bromide and various bromohexanes, depending on the degree of bromination. In your example, you have excess hexane and you will get monobromohexane. With excess bromine water and a few drops of hexane, you will get various polybromohexanes, which will appear as dense globules that sink to the bottom of your bromine water.
What causes water to turn brown?
Bromine water is a strong oxidizing agent and pretty much acidic. so practically anything that can cause Br2 to be reduced since the presence of Br2 that cause the water turn brown color. unsaturated hydrocarbons like alkene, alkyne, phenol, phenylamine, acetyl, aldehyde and some carbohydrate.
What does it mean when Br2+H2O is written?
1)Br2 aq is different and Br2+H2o is different.whenever Br2 +H2o is written it means that it is having more reactivity as compare to Br2 aq.
What compounds react well with bromine?
The most common compounds that react well with bromine water are phenols, alkenes, enols, the acetyl group, aniline and glucose. In addition, bromine water is commonly used to test for the presence of an alkene which contains a double covalent bond which reacts with the bromine water which changes its colour from an i.
How to make bleach with sodium bromide?
A much safer way of preparing it is by adding sodium bromide into a container with hydrochloric acid. To this, sodium hypochlorite is added. This is the active chemical in bleach. It is really important when shaking to make sure the container is sealed tightly.
What is the formula for bromine water?
Bromine water, also called Bromide bromate solution or Bromine solution with the chemical formula Br 2. The molecular weight of bromine water is 159.81 and the density is 1.307 g/mL. Bromine water is a yellow mixture solution with high oxidizing property, prepared by dissolving diatomic bromine (Br 2) in water (H 2 O).
How to make bromine water?
Bromine water solution can be prepared in the chemistry lab by direct mixing of fumes of bromine and water. But it is not safe, so the more convenient method of preparation of bromine water solution is breaking of sodium bromide (NaBr) in the presence of bleach and hydrochloric acid (HCl).
What is the reaction between phenols and bromine water?
Phenols. Phenols undergo substitution reactions in the presence of bromine water to give a brominated compound. During the process, bromine water is decolourized and gives white precipitate. The mechanism of the reaction is given below, 4. Aniline. Aniline or phenylamine reacts with bromine water.
What is the most common compound to undergo a bromine water test?
Enols, alkenes, aniline, glucose, phenols and acetyl groups are the most common compounds to undergo bromine water test. The test also identifies the presence of an aldehyde group in the compound. During the process, the colour of the bromine water changes from yellow to colourless.
What is bromine water used for?
Bromine water is used to identify the functional group present in the organic compound by halogenation mechanism.
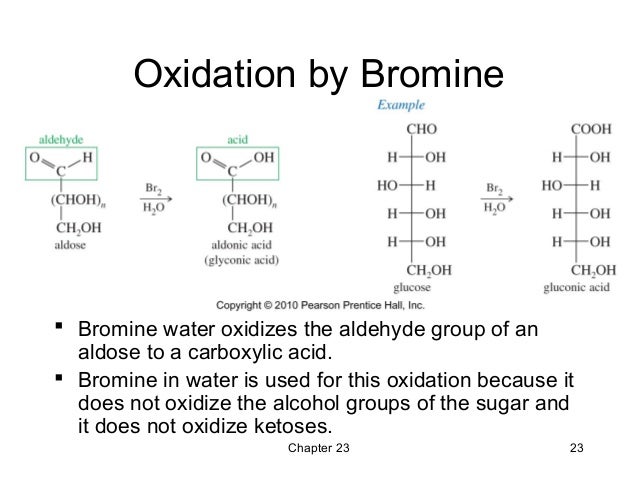
What is the reaction of aldehyde and bromine?
Aldehyde reacts with bromine water and undergoes an oxidation reaction to give a colourless solution.
Does alkane react with bromine?
On the other hand, alkane doesn’t react with bromine water and the colour of the bromine water remains the same.