“Mass defect” refers to the difference between the mass of the nucleons (protons + neutrons) in a nucleus when weighed separately and the mass of the nucleus when it’s put together. This difference is important because this missing mass is converted to energy using E=mc2that’s used to hold the nucleus together.
How do I calculate the mass defect?
- Because we often deal with isotopes, ensure that you are using the correct number of protons and neutrons in your calculations. Thanks! ...
- Use the atomic mass units while calculating the masses. ...
- Double check your arithmetic in addition to your values for the masses of a proton and neutron. ...
How do you calculate mass defect?
How do you calculate mass defect? Upvote 3 Downvote 1. Share. Answer it. To calculate the mass defect: add up the masses of each proton and of each neutron that make up the nucleus, subtract the actual mass of the nucleus from the combined mass of the components to obtain the mass defect.
How to calculate the mass defect?
- What is the question asking you to do? ...
- (i) What data have you been given? ...
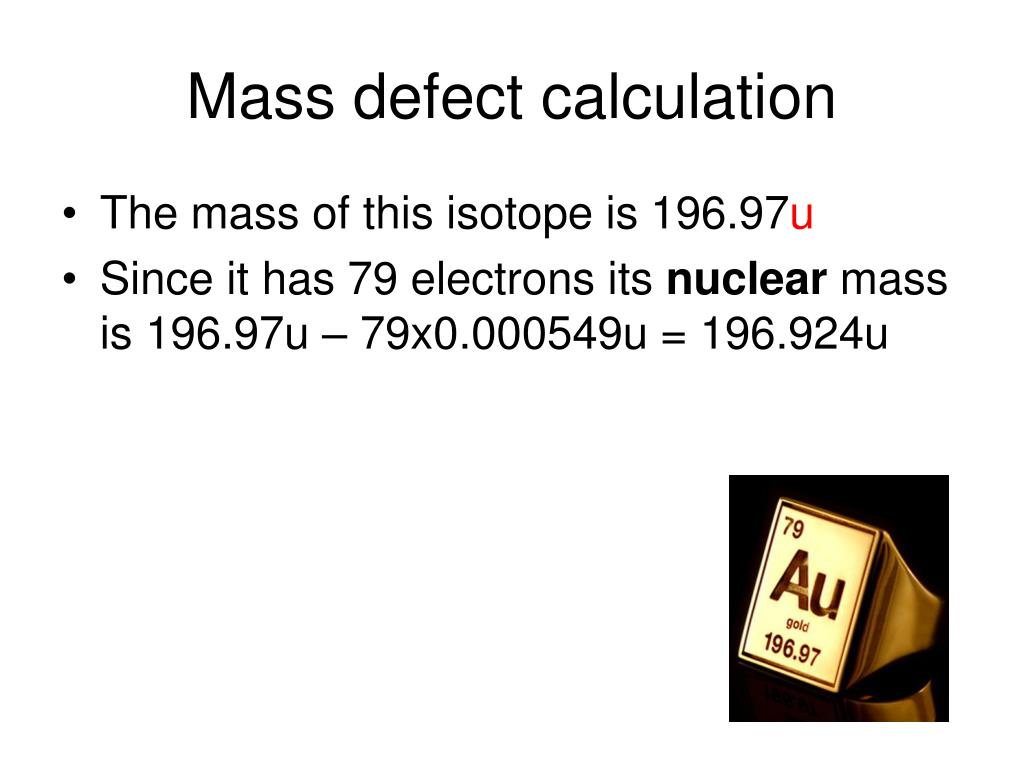
- Calculate the mass defect For 238 U: Z = 92 (from Periodic Table ) A = 238 (from data in question) From the data sheet proton mass = 1.673 × ...
- Is your answer plausible? ...
- State the solution to the problem Mass defect for uranium-238 is 3.983 × 10 -25 kg.
What is mass defect and why is it important?
What are 3 uses for nuclear reactions?
- Agriculture and Food. In many parts of the world, agricultural workers use radiation to prevent harmful insects from reproducing.
- Medical.
- Space Exploration.
- Water Desalination.
What is a mass defect?
Careful measurements have shown that the mass of a particular atom is always slightly less than the sum of the masses of the individual neutrons, protons, and electrons of which the atom consists. The difference between the mass of the atom and the sum of the masses of its parts is called the mass defect (Δm).
Why is mass defect important for nuclear reactions?
Mass defect is important academically because it disproved a previously held tenet of chemistry, namely that mass and energy could not be converted into each other. Mass defect is important practically because it is a factor in nuclear reactions (reactions in which elements are converted into other elements).
Why do we need mass defect?
Nuclear binding energy is used to determine whether fission or fusion will be a favorable process. The mass defect of a nucleus represents the mass of the energy binding the nucleus, and is the difference between the mass of a nucleus and the sum of the masses of the nucleons of which it is composed.
What is mass defect of a nucleus?
The mass defect of a nucleus is the difference between the total mass of a nucleus and the sum of the masses of all its constituent nucleons. The binding energy (BE) of a nucleus is equal to the amount of energy released in forming the nucleus, or the mass defect multiplied by the speed of light squared.
How does mass defect affect nuclear stability?
The mass equivalent of mass defect is converted into energy which is given by Einstein's energy equation. Where C = speed of light in free space. This energy holds the nucleons together in the nucleus called nuclear binding energy. Hence nuclear stability is proportional to the mass defect.
What is responsible for mass defect?
b. The mass defect comes from the electrons of the newly formed atom.
How do you do a mass defect?
To calculate the mass defect:add up the masses of each proton and of each neutron that make up the nucleus,subtract the actual mass of the nucleus from the combined mass of the components to obtain the mass defect.
What is mass defect in mass spectrometry?
Mass defect (mass spectrometry) In nuclear physics, the mass defect is the difference in the mass of a composite particle and the sum of the masses of its component parts. In mass spectrometry the mass defect is defined as the difference between the exact mass and the nearest integer mass.
What is the difference between mass excess and mass defect?
Mass excess (aka mass defect) is equal to the difference between atomic mass and the atomic number times the atomic mass unit. Mass excess is convenient to use in calculations of nuclear decay and reactions energetics. It is frequently used to tabulate atomic masses.
What is mass defect explain Class 12?
The mass defect is the difference between the sum of mass of component particles and the mass of the atom. The greater the mass defect for an atom, the more stable is the atom. Stability of the atom is determined by the binding energy of an atom, it is given by- E=Δmc2.
What is mass defect of a nucleus class 12?
It is observed that the mass of the nucleus is less than the sum of the masses of constituent nucleons in the free State. The difference between the actual mass of the nucleus and the sum of masses of constituent nucleons is called the mass defect.
What is mass defect how it is related to binding energy?
The energy equivalent to mass defect is used in binding the nucleons and is called the binding energy. If Δm is the mass defect of a nucleus, then according to Einstein's mass energy relation, Binding energy = Δmc2( in joule ).
How to calculate mass defect?
In calculating the mass defect, it is important to use the full accuracy of mass measurements because the difference in mass is small compared to the mass of the atom. Rounding off the masses of atoms and particles to three or four significant digits prior to the calculation will result in a calculated mass defect of zero.
What is the energy equivalent to the mass defect of a nucleus?
The energy equivalent to the mass defect of a nucleus is known as the binding energy, which is the energy required to dismantle the nucleus into its individual constituent nucleons or, alternatively, the energy released when the nucleons come together to form the nucleus.
What is the difference between the mass of an atom and the mass of its parts?
The difference between the mass of the atom and the sum of the masses of its parts is called the mass defect (Δm).
Why is it important to use the full accuracy of mass measurements?
In calculating the mass defect it is important to use the full accuracy of mass measurements because the difference in mass is small compared with the mass of the atom. Rounding off the masses of atoms and particles to three or four significant digits before the calculation will result in a calculated mass defect of zero.
How much energy is available for fission?
The binding energy per nucleon and the mass defect Δm predicts that about 1 MeV per nucleon is available for producing energy. But not all of the energy is available for power production since the fission process yields fission fragments, gamma rays, and beta decay particles. Also neutrinos are released with energy unavailable for heat. To find the amount of energy available from uranium fissions, the binding energy of the U-235 isotope, which is about 7.5 MeV per nucleon, can be used. Most of the fission products in the 80–150 mass number range have 8.4 MeV per nucleon binding energies. The difference per nucleon is therefore 0.9 MeV. When the U-235 is bombarded by the neutron, a compound nucleus (U-236) is formed so that the number of particles (236) can be multiplied by the excess binding energy per nucleon (0.9 Mev) or approximately 200 MeV. Table III gives the energy distribution for U-235 thermal neutron fissions. The kinetic energy of the fission fragments and the instantaneous gamma rays are available for producing heat in the fuel material for energy production. The fission product gamma and beta decay energies can be recovered partially during the lifetime of the fuel. To understand how much energy is available from fission, 1 g of U-235 if fully consumed by fission would produce approximately 1 million watts, or 1 MW of energy.
What is nuclear mass defect?
The nuclear mass defect is a fundamental property of a nucleus and is a fixed value corresponding to a certain amount of binding energy for that nucleus. Mass defect and binding energy are important factors in the energy involved in nuclear reactions. Looking at how mass defect and binding energy change from one element to another will make the relationship between mass defect, binding energy, and nuclear energy more apparent. A plot of nuclear mass defect versus mass number for different elements is shown in Figure 1.
What is the mass defect of the nucleus?
Mass defect is associated with the binding energy of the nucleus. It is a fundamental property of the nucleus and the principle behind nuclear energy. Mass defect has also entered into the mass spectrometry terminology with the availability of high resolution mass spectrometry and has found application in mass spectral analysis. In this application, isobaric masses are differentiated and identified by their mass defect. What is the relationship between nuclear mass defect and mass defect used in mass spectral analysis, and are they the same?
What is the difference between nominal and monoisotopic mass?
It is equivalent to mass number expressed in mass unit [ 21] and they are both represented by the same symbol, A, here. Therefore, chemical mass defect is the difference between the monoisotopic mass and a whole number mass, which may not be the closest integer mass. Nominal mass is the closest integer mass to the monoisotopic mass for low molecular weight compounds but this is not necessarily true at higher masses where the difference between monoisotopic and nominal masses can be quite large [ 22 ]. For example, polystyrene, C 4 H 9 (C 8 H 8) 100 H, has a nominal mass of 10458 u and monoisotopic mass of 10464.338 u. Chemical mass defect as defined in Equation 6 is what is currently used in mass spectral analysis. Because homologous series constitute a large portion of the compounds found in petroleum samples, Kendrick introduced a new mass scale based on CH 2 = 14.0000 ( 12 C scale based masses are multiplied by 14.0000/14.01565 in order to be converted to the Kendrick mass scale). On this mass scale, the repeating mass of methylene does not change the mass defect and all of the compounds in a homologous series that belong to the same class and type will have the same chemical mass defect. Plotting chemical mass defect versus nominal mass will help visualize all of the compounds present in the spectrum in a way that would not be possible by just viewing the spectrum. Figure 3 shows an example of such a plot for peaks from the high-resolution mass spectrum of a crude oil sample [ 23 ]. Compounds belonging to the same class and type but with different number of CH 2 groups will fall on a horizontal line on this plot. Similarly, compounds of the same class but different type differ by two hydrogens and will fall on horizontal lines separated by the mass defect of H 2. Compounds belonging to different classes are now readily identified because their chemical mass defect will be displaced vertically from each other. Visualization of a complex mass spectrum is simplified by using a simple two-dimensional graphical display of the data based on chemical mass defect. Patterns are recognizable on the plot, and the outlier data are easily identified. Identification of a few compounds on the plot, at least one from each class, is the key to identifying the majority of the compounds. Such a plot has been used for analyzing data from a single high resolution mass spectrum of a crude oil sample containing several thousand ion peaks [ 24 ]. Class and type assignment for so many compounds in the sample was accomplished by taking advantage of their chemical mass defect, a task that would be difficult to achieve in the absence of such a powerful data interpretation strategy. A CH 2 based mass scale is historically the first one used for the analysis of crude oil samples by mass defect. Other mass scales ( 16 O- and H 2 -based for example) have also been used to plot data on two-dimensional plots similar to the one shown in Figure 3, and are useful for environmental samples [ 25 ]. The use of more than two mass scales for graphical visualization of data on higher-order plots makes data interpretation easier and increases the number of assigned chemical formulas [ 26 ].
What is a mass tag?
Mass tags contain atoms with large chemical mass defects per nucleon . The presence of a mass tag in a compound will shift its ion signal from the crowded areas of the spectrum to the gaps where it can be easily detected and identified [ 13 ]. Tagging proteins, for example, has been used to improve protein sequencing and identification [ 35 ]. Fluorinated compounds have long been popular for mass calibration and as internal standards because they have chemical mass defects that are different from naturally occurring compounds and less likely to interfere with the analysis [ 36 ].
How did mass spectrometry help in petroleum analysis?
Petroleum samples contain many compounds with the same nominal mass but with different elemental compositions that could be identified by high resolution instruments. A goal of petroleum analysis was to identify compounds based on their class, type, and the degree of alkylation . Compound class is collectively defined as all of elemental compositions with the same heteroatom content. Compounds with the same heteroatom but with various numbers of hydrogen in their empirical formula belong to different compound types. Compound type arises from different number of double bonds or rings in the molecule. Within the same class and type, there are many compounds with varying degrees of alkylation, which differ only in the numbers of methylene (CH 2) groups in their formula. They are commonly known as homologous series. Presence of a large number of different molecules made the interpretation of the mass spectra of petroleum samples a difficult task. The high-resolution spectra of such samples with many resolved peaks at the same nominal mass demanded a new approach for data interpretation. A data interpretation strategy was developed based on the chemical mass defect. Mass defect was defined as the difference between the accurate mass of the ion in question and a reference hydrocarbon ion with the same nominal mass [ 10 ]. This approach was used to identify several new compound classes and types not reported before.
Why are atomic masses not whole numbers?
Why are atomic masses not whole numbers? The quest to answer this question and to evaluate the divergence of atomic masses from whole numbers led to the concepts of nuclear mass defect and binding energy. Later on, chemical mass defect played an important role in mass spectral analysis. Nuclear and chemical mass defects are both caused by the strong nuclear force. However, despite their common origin and close relationship, they are different concepts. Nuclear mass defect is an absolute parameter whereas chemical mass defect is a relative value. Nuclear mass defect and binding energy are intrinsic properties and are fixed values for a certain atom. On the other hand, chemical mass defect is not a fixed value and depends on the mass scale. Nuclear mass defect reflects a physical property whereas chemical mass defect is not a physical property and is based on a convention that carbon has a mass defect of zero. Considering the differences between the nuclear and chemical mass defects, it is proposed to refer to the latter as mass excess. This will eliminate confusion surrounding the use of the term mass defect especially among different disciplines and will harmonize the terminologies used in nuclear physics and mass spectrometry.
What is the energy equivalent of the nuclear mass defect?
The energy equivalent of the nuclear mass defect is known as the nuclear binding energy. In other words, binding energy is the energy released with the formation of a nucleus from its nucleons, or is the energy required to break a nucleus into its individual components.