Period 6 Element (Periodic Table)
| group | 1 | 2 | 5 | |
| period | 1 | 1 H | ||
| 2 | 3 Li | 4 Be | ||
| 3 | 11 Na | 12 Mg | ||
| 4 | 19 K | 20 Ca | 23 V |
What element is found in Group 3 and period 6?
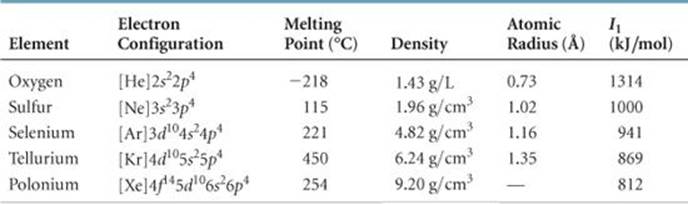
Group 6A (or VIA) of the periodic table are the chalcogens : the nonmetals oxygen (O), sulfur (S), and selenium (Se), the metalloid tellurium (Te), and the metal polonium (Po). The name "chalcogen" means "ore former," derived from the Greek words chalcos ("ore") and -gen ("formation").
What element name is Group 6 period 6?
There are 4 elements in group 6 in periodic table. Atomic Number. Chemical Symbol. Element Name. Group. Period. 24. Cr. Chromium.
Which elements are main group elements?
- nonmetal: C; semimetals: Si and Ge; metals: Sn and Pb
- Pb is most stable as M 2+.
- C is most different.
- C and P are most similar in chemistry.
What is the element in period 6 and group 12?
Group 12 element. Group 12, by modern IUPAC numbering, is a group of chemical elements in the periodic table. It includes zinc (Zn), cadmium (Cd) and mercury (Hg). The further inclusion of copernicium (Cn) in group 12 is supported by recent experiments on individual copernicium atoms.
What is the name of the group of elements in Group 6?
Group 6, numbered by IUPAC style, is a group of elements in the periodic table. Its members are chromium, molybdenum, tungsten, and seaborgium. These are all transition metals and chromium, molybdenum and tungsten are refractory metals.
What are the main group elements?
The main group elements are groups 1, 2, and 13-18 on the periodic table. The main group elements are the chemical elements belonging to the s-block and p-block on the periodic table. These are elements in group 1 and group 2 (s-block) and groups 13 through 18 (p-block).
Which element is a main group metal?
Group 1 (Alkali Metals) The alkali metals are the series of elements in Group 1 of the periodic table (excluding hydrogen in all but one rare circumstance). The series consists of the elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr).
Where are main group elements on the periodic table?
The main group elements are those in the s and p blocks of the periodic table, as shown in Figure 8.2. 1. Figure 8.2.
How many main group elements are there?
The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons.
What are the 5 main groups of the periodic table?
5 Element FamiliesAlkali metals.Alkaline earth metals.Transition metals.Halogens.Noble gases.
What are the 2 main groups on the periodic table?
The metals are on the bottom left in the periodic table, and the nonmetals are at the top right. The semimetals lie along a diagonal line separating the metals and nonmetals. The elements are arranged in a periodic table, which is probably the single most important learning aid in chemistry.
Is iron a main group element?
Group 8 is a group (column) of chemical elements in the periodic table. It consists of iron (Fe), ruthenium (Ru), osmium (Os) and hassium (Hs). They are all transition metals....Group 8 element.IronCopperZincGalliumGermanium6 more columns
What is a main group element in period 3?
The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block.
What are the 4 main groups in the periodic table?
There are multiple ways of grouping the elements, but they are commonly divided into metals, semimetals (metalloids), and nonmetals. You'll find more specific groups, like transition metals, rare earths, alkali metals, alkaline earth, halogens, and noble gasses.
What do you call the main groups of the periodic table?
The columns with B (IB through VIIIB) are called the transition elements. The columns with A (IA through VIIIA) are called the main group elements. The elements can also be divided into two main groups, the metals and the non-metals.
What are main group transition and inner transition elements?
Group 18 elements are called noble gases. Groups 1, 2, and 13-18 are the representative elements (or main-group elements). Groups 3-12 are called the transition metals. The two rows at the bottom of the table are called inner transition metals.
What are the main group elements?
In chemistry and physics, the main group elements are any of the chemical elements belonging to the s and p blocks of the periodic table. The s-block elements are group 1 ( alkali metals) and group 2 ( alkaline earth metals ).
Which elements should be included in the main group?
Some scientists believe zinc, cadmium, and mercury should be included as main group elements. Others believe group 3 elements should be added to the group. Arguments may be made for including the lanthanides and actinides, based on their oxidation states.
How many oxidation states does a s-block have?
The s-block elements usually have one oxidation state (+1 for group 1 and +2 for group 2). The p-block elements may have more than one oxidation state, but when this happens, the most common oxidation states are separated by two units.
Is the D block a main group element?
Traditionally, the d-block elements have not been considered to be main group elements. In other words, the transition metals in the middle of the periodic table and the lanthanides and actinides below the main body of the table are not main group elements.
State of Matter
group 1: Hydrogen; H, Lithium; Li, Sodium; Na, Potassium; K, Rubidum; Rb, Cesium; Cs, Francium; Fr
Metallic Character
Group 1: All the Group 1 elements besides Hydrogen are silvery-coloured metals and Alkali Metals. The metallic character increases as you go down the group.
Interesting facts
Lithium is used in batteries. Sodium will explode when added to water. Potassium is one of the most abundant elements found in the earth's crust. Francium is the most reactive metal.
Lewis Dot Structure
For Groups on the periodic table, the Lewis Dot structure will remain the same for each element. Since the number of valence electrons is the same for all group 1 elements, the Lewis Dot structure is also the same. Lewis Dot structure is created from the number of valence electrons.
General Research- bonding behavior
Period 6: Atomic Radii: Decreases as you go left to right on the period
How many groups are there in the periodic table?
Groups are the vertical columns on the periodic table. There are total 18 vertical columns on periodic table. Hence there are 18 groups. The elements lying in the same groups show similar chemical properties and they also have same number of valence electrons.
What is the first group of elements in the periodic table?
Group 1: Alkali metals group. Alkali metals group is the very first group (group 1) on the periodic table. The elements included in the Alkali metals group are; Lithium (Li)
Why are the elements in the bottom two rows of the periodic table included in group 3?
The elements in the two bottom rows of the periodic table are also included in these groups. They are placed in the two separate rows at the bottom because they show few different properties. Actually, the elements in the bottom rows are the extension of group 3 only. So they are included in group 3. But as these elements have few different ...
What is an example of group 18?
Example of group 18. All the elements of group 18 are chemically inert (that means they do not easily react with other elements). And all the elements of group 18 have a complete octet (that means they have 8 electrons in their outer shell).
Overview
d-block elements
Lutetium (/ljuːˈtiːʃiəm/ lew-TEE-shee-əm) is a chemical element with the symbol Lu and atomic number 71. It is the last element in the lanthanide series, which, along with the lanthanide contraction, explains several important properties of lutetium, such as it having the highest hardness or density among lanthanides. Unlike other lanthanides, which lie in the f-block of the periodic table, this element lies in the d-block; however, lanthanum is sometimes placed on the d-…
Properties
This period contains the lanthanides, also known as the rare earths. Many lanthanides are known for their magnetic properties, such as neodymium. Many period 6 transition metals are very valuable, such as gold, however many period 6 other metals are incredibly toxic, such as thallium. Period 6 contains the last stable element, lead. All subsequent elements in the periodic table are radioactive. After bismuth, which has a half-life or more than 10 years, polonium, astatine, and rad…
Atomic characteristics
Chemical element Block Electron configuration 55 Cs Caesium s-block [Xe] 6s 56 Ba Barium s-block [Xe] 6s 57 La Lanthanum f-block [Xe] 5d 6s 58 Ce Cerium f-block [Xe] 4f 5d 6s 59 Pr Praseodymium f-block [Xe] 4f 6s 60 Nd Neodymium f-block [Xe] 4f 6s 61 Pm Promethium f-block [Xe] 4f 6s 62 Sm Samarium f-block [Xe] 4f 6s 63 Eu Europium f-block [Xe] 4f 6s 64 Gd Gadolinium f-block [Xe] 4f 5d 6s 65 Tb Terbium f-block [Xe] 4f 6s 66 Dy Dysprosium f-block [Xe] 4f 6s 67 Ho …
Chemical element Block Electron configuration 55 Cs Caesium s-block [Xe] 6s 56 Ba Barium s-block [Xe] 6s 57 La Lanthanum f-block [Xe] 5d 6s 58 Ce Cerium f-block [Xe] 4f 5d 6s 59 Pr Praseodymium f-block [Xe] 4f 6s 60 Nd Neodymium f-block [Xe] 4f 6s 61 Pm Promethium f-block [Xe] 4f 6s 62 Sm Samarium f-block [Xe] 4f 6s 63 Eu Europium f-block [Xe] 4f 6s 64 Gd Gadolinium f-block [Xe] 4f 5d 6s 65 Tb Terbium f-block [Xe] 4f 6s 66 Dy Dysprosium f-block [Xe] 4f 6s 67 Ho …
s-block elements
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C (82 °F), which makes it one of only five elemental metals that are liquid at (or near) room temperature. Caesium is an alkali metal and has physical and chemical properties similar to those of rubidium and potassium. The metal is extremely reactive and pyrophoric, reacting with water even at−116 °C (−177 °F). It is the least el…
f-block elements (lanthanides)
The lanthanide or lanthanoid (IUPAC nomenclature) series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium. These fifteen elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements.
The informal chemical symbol Ln is used in general discussions of lanthanide chemistry. All but …
p-block elements
Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray other metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy. Both discovered the new element in residues of sulfuric acid production.
Approximately 60–70% of thallium production is used in the electronics industry, and the remain…
Biological role
Of the period 6 elements, only tungsten is known to have any biological role in organisms. However, gold, platinum, mercury, and some lanthanides such as gadolinium have applications as drugs.