Why is fluorine an F ion?
Since the electron charge is -1, the F atom must gain an electron to become the Fˉ ion. The usual, useful notion is that one “extra” electron is localized to the fluorine atom, causing the atom to become a fluoride ion.
What is the charge of fluorine when it gains an electron?
If a fluorine atom gains an electron, it becomes a fluoride ion with an electric charge of -1. Click to see full answer. Likewise, does fluorine gain or lose electrons? It can lose one of its electrons, making it an ion. It now has more positive protons than electrons so it has an overall positive charge.
What happens when an atom gains an electron?
If atoms gain electrons, they become negative ions, or anions. Consider the example of fluorine (see Figure below). A fluorine atom has nine protons and nine electrons, so it is electrically neutral.
What is the electron configuration for a fluorine bond?
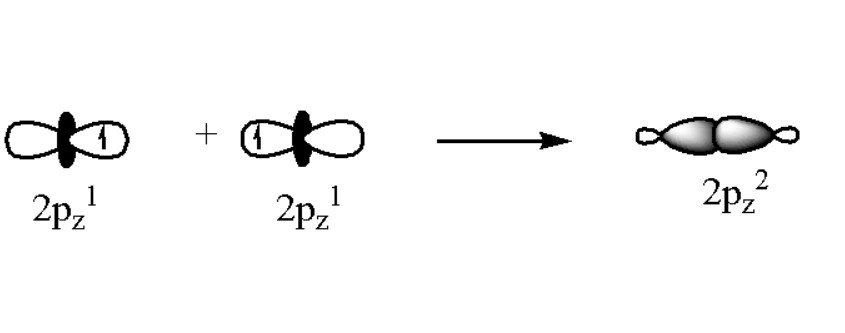
Since fluorine bonds covalently, the electron is “shared” between the fluorine atom and the atom to which it is bonded, but the electron will be more present near the fluorine atom. (In F₂, the sharing is necessarily equal: each F atom has an “extra” electron from the other, so the electrons are equally localized at the F atoms.)
What happens to fluorine when it gains an electron?
Fluorine, F It gains an electron from another atom in reactions, forming a fluoride ion, F -. Note that the atom is called fluorine, but the ion is called fluoride. A fluoride ion has the same electronic structure as a neon atom (Ne).
Does fluorine lose or gain electrons?
When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge.
What happens when an electron is gained?
If an atom or molecule gains an electron, it becomes negatively charged (an anion), and if it loses an electron, it becomes positively charged (a cation). Energy may be lost or gained in the formation of an ion.
Why did fluorine gains one electron?
Atomic structure - the atom-fluorine gaining electrons. Fluorine needs one electron to fill the outer energy level. It will get one electron from another atom.
When fluorine gains an electron to become the ion fluoride Its charge is?
-1A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If a fluorine atom gains an electron, it becomes a fluoride ion with an electric charge of -1.
Which elements gain or lose electrons?
In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Hydrogen is an exception, as it will usually lose its electron. Metalloids and some metals can be can lose or gain electrons.
Will fluorine form a cation or anion?
Does it form a cation or an anion? Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now has one more negatively charged electron than positively charged protons. This is called an anion because the element gained electron and is a negatively charged ion.
What type of ion will fluorine form?
A fluorine atom has nine protons and nine electrons, so it is electrically neutral. If a fluorine atom gains an electron, it becomes a fluoride ion with an electric charge of -1.
What is being formed when an atom gains an electron?
When an atom loses or gains an electron, ions are formed. If an atom loses electron it forms positive charge and if gains electron it forms negative charge. An atom which carries charge on it is known as ion.
When fluorine gains 1 more electron what is the new electron configuration that it will have *?
The electron configuration of a neutral fluorine atom is 1s22s22p5 . When a fluorine atom gains one electron, it becomes a fluoride ion with 10 negatively charged electrons and 9 positively charged protons, which gives it a 1− charge. The electron configuration of a F− ion is 1s22s22p6 .
Is fluorine stable or unstable?
Reactions of Fluorine It is very unstable and reactive since it is so close to its ideal electron configuration. It forms covalent bonds with nonmetals, and since it is the most electronegative element, is always going to be the element that is reduced.
When fluorine becomes an ion It has the same electron number as which noble gas?
neonNonmetals will typically form ions by gaining enough electrons to become isoelectronic with the nearest noble gas. For example, fluorine has 7 valence electrons and is one electron away from being isoelectronic with neon, which has a stable noble gas electron configuration (see Figure above).
What happens when fluorine bonds?
Since fluorine bonds covalently, the electron is “shared” between the fluorine atom and the atom to which it is bonded, but the electron will be more present near the fluorine atom. (In F₂, the sharing is necessarily equal: each F atom has an “extra” electron from the other, so the electrons are equally localized at the F atoms.)
Why do positive ions lose electrons?
This is actually wrong since the energy required to remove an electron from an atom to form a positive ion is always endothermic (Ionization energy is always positive). If you like the ISOLATED neutral atom is more stable than the positive ion. Positive ions will only form in conjunction with another particle , usually another neutral atom that can accept the electron to form a negative ion. It is the overall process by which one atom acts as an electron donor and the other acts as an electron accepto
What is it called when an electron is added to an atom?
After loosing or gaining electron it would be an atom but we call it (ion) if electron is removed its CATION and if electron is added its called ANION .
Is fluoride reduced?
Well, fluoride ion has been reduced…and we can represent this by the redox equation…
Can an atom be an ion?
Now, first of all, an atom can’t be called an ion until it loses and electron. So, your question should have been ‘ What is an atom that loses its electron’. Now, for your question, an atom which loses its electron is called a Cation since it has more protons then electrons after losing it, as per Chemistry. Now as per Physics, you can’t called this an ion, you need to call it a positively charged atom.
Why do atoms gain electrons?
Atom gain electrons to complete a octate of valence shell. At that time no. of protons in an atom remains same and due to gaining of electrons negative charge on an atom increases.
What happens when a neutral atom gains electrons?
If a neutral atom gains electrons, it becomes an anion or it keeps a negative charge and it tries to make ionic bond with cation.
Why is an atom neutral?
An atom is in general electrically neutral because the number of positively charged protons inside the nucleus of an atom is equal to the numbers of negatively charged electrons revolving outside the nucleus. When an atom gains a electron it has one unit of exces negative charge. So the atom becomes negatively charge. That means it becomes anion.
What happens when an atom is a negative ion?
The atom becomes an anion (a negative ion), and can be attracted to positive charges (such as those of atoms that have lost electrons and become cations). If these charged atoms are allowed to combine, the electron transfer can be essentially complete, forming an ionic bond; or the electron (s) can be shared between the atoms, ...
What is the charge of an atom?
An atom is in general neutral ie the total amount of positive charge inside the nucleus and the total amount of negative charge of the electrons outside the nucleus is equal and it makes the atom neutral. When a neutral atom gains electrons ie it has more negative charge than positive charge, the atom becomes a negative ion, an anion, ...
How fast is an electron?
Approximately speed of an electron around the nucleus is about 2.2 k (2200) kilometres per second. 3.8K views.
When a neutral atom gains electrons, it has more negative charge than positive charge?
When a neutral atom gains electrons ie it has more negative charge than positive charge, the atom becomes a negative ion, an anion, which under the action of an electric field moves to the anode. The Rock admits this was the best decision he ever made. The big companies don't want you to know his secrets.