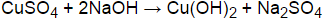What is the objective of reaction between CuSO4 and NaOH?
What is the objective of reaction between CuSO4 and NaOH? The reaction between CuSO4 and NaOH is a double displacement reaction that forms Na2SO4 and Cu(OH)2. Copper (II) hydroxide will precipitate from the solution and appears as a pale blue solid.
Is NaOH a solute or solvent?
mass of NaOH = 1g NaOH (solute) and Volume of solution= 100 mL. get the volume of NaOH; density of NaOH = 1.5g/mL. Volume NaOH = 1g/ 1.5g-mL^- = 0.67 mL. Answer: volume of water ( solvent) = 100- 0.67 NaOH = 99.33 mL H2O
What happens when NaOH reacts with CH3COOH?
Acetic acid, CH3COOH, will react with sodium hydroxide, NaOH, to produce sodium acetate, CH3COONa, and water. The unbalanced chemical equation that describes this neutralization reaction. Which of the following is the net ionic equation for the neutralization of CH3COOH with NaOH? Writing the Equation.
Does Cu react with NaOH?
When Cu^2+ i.e.the solution of copper salt reacts with aqueous solution of sodium hydroxide(NaOH), the light blue coloured precipitate is formed. The reaction equation is as follows: Cu^2+(aq) +2 NaOH(aq) => Cu(OH)2(s) +2 Na+(aq)
What happens if you add too much NaOH to CuSO4?
If sodium hydroxide solution is then added, a transition metal hydroxide is formed. These are insoluble. Copper solutions form a blue precipitate with sodium hydroxide.Jan 24, 2020
What happens when you add excess NaOH?
A few drops of dilute sodium hydroxide solution react to form a white precipitate with aluminium ions, calcium ions and magnesium ions. However, if excess sodium hydroxide solution is added: the aluminium hydroxide precipitate dissolves to form a colourless solution.
What kind of reaction is NaOH CuSO4?
0:001:58How to Balance CuSO4 + NaOH = Cu(OH)2 + Na2SO4 - YouTubeYouTubeStart of suggested clipEnd of suggested clipTo bounce this equation copper ii sulfate plus sodium hydroxide yields copper two hydroxide plusMoreTo bounce this equation copper ii sulfate plus sodium hydroxide yields copper two hydroxide plus sodium sulfate let's add the atoms up on both sides of the equation. So I have one copper atom here on
What happens when copper reacts with NaOH?
If copper exists in the form of salt, and it is called precipitation reaction. Cu2+(aq)+2NaOH(aq)→ Cu(OH)2(s)+2Na+(aq), which gives a very beautiful blue precipitate.
What precipitate dissolves in excess NaOH?
Precipitate testsMetal ionResult on adding NaOH or NH 3 solutionEffect of adding excess NaOH solutionaluminium, Al 3+white precipitatewhite precipitate dissolves and a colourless solution formszinc, Zn 2+white precipitatewhite precipitate dissolves and a colourless solution forms4 more rows
Is cuso4 soluble in NaOH?
On adding sodium hydroxide to copper sulphate solution, a pale blue precipitate of copper hydroxide is formed which is insoluble in excess of sodium hydroxide.Jun 4, 2014
How does it react with CuSO4 solution?
When NH3 solution is added to CuSO4 solution drop by drop, ammonia ions completely react with copper sulphate and precipitates of copper hydroxide are formed. But when we add excess of ammonia, the precipitate dissolves and a soluble complex is formed.
Is NaOH CuSO4 endothermic or exothermic?
This reaction is highly exothermic, which means it releases heat in the form of energy.Apr 24, 2021
What is the net ionic equation for CuSO4 and NaOH?
3:484:09How to Write the Net Ionic Equation for CuSO4 + NaOH = Cu(OH)2 + ...YouTubeStart of suggested clipEnd of suggested clipSo this is the net ionic equation for cuso4 plus NaOH copper ii sulfate plus sodium hydroxide.MoreSo this is the net ionic equation for cuso4 plus NaOH copper ii sulfate plus sodium hydroxide.
What type of reaction is copper hydroxide to copper oxide?
decomposition reactionSTEP #3 - Conversion of Copper (II) Hydroxide to an Insoluble Oxide: Heat applied to the copper (II) hydroxide causes black, insoluble copper (II) oxide, Cuo, to form. This reaction is a decomposition reaction.
Does NaOH dissolve copper?
0:010:39Reaction of copper(II) ions with NaOH - YouTubeYouTubeStart of suggested clipEnd of suggested clipCopper ions react with sodium hydroxide to form a blue precipitate of copper two hydroxide. ThisMoreCopper ions react with sodium hydroxide to form a blue precipitate of copper two hydroxide. This precipitate does not dissolve in excess sodium hydroxide solution.
What happens when you heat copper hydroxide?
Heating copper hydroxide produces copper oxide, CuO, a black solid. Copper oxide dissolves in acid, regenerating the copper (II) ion, which once again binds to water.
Does copper sulfate react with sodium hydroxide?
Well, the copper (II) sulfate would react with sodium hydroxide in solution; this gives insoluble copper (II) hydroxide and leaves sodium sulfate in solution. I'm not really sure about the gas, though...
Can sulfates dissolve in water?
No because all group one metals are completely soluble in water, and thus dissociate. Also most sulfates are soluble in water meaning that the reaction you propose would not happen.