What is Hund's rule in simple language?
in simple language we can say,Hund's rule states that the lowest energy electron configuration, the ground state, in any electron subshell is the one with the greatest number of parallel electron spins.
What are the three Hund's rules?
Hund's rules. The three rules are: For a given electron configuration, the term with maximum multiplicity has the lowest energy. The multiplicity is equal to , where is the total spin angular momentum for all electrons. Therefore, the term with lowest energy is also the term with maximum . For a given multiplicity,...
What is Hund's first rule in chemistry?
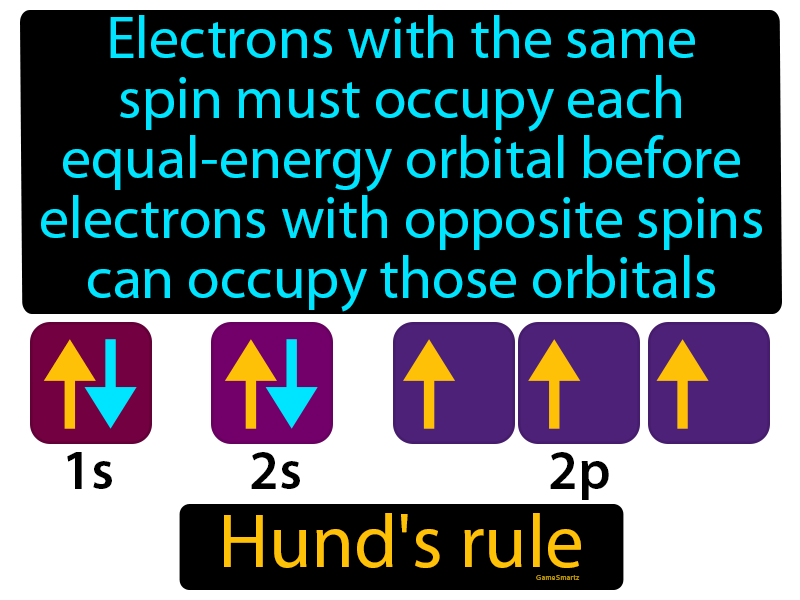
Hund's first rule states that the lowest energy atomic state is the one that maximizes the total spin quantum number for the electrons in the open subshell. The orbitals of the subshell are each occupied singly with electrons of parallel spin before double occupation occurs.
What is Hund's rule for electron configuration?
All of the electrons in separately occupied orbitals have an equivalent spin (to maximize total spin). This rule is fairly reliable (with occasional failures) for the determination of the state of a given excited electronic configuration. Hund's rule is also known as the rule of maximum multiplicity.
What is Hund rule give its example?
example of hund's rule (example of hund's rule of maximum multiplicity): For example, a nitrogen atom's electronic configuration would be 1s 2 2s 2 2p 3 . The same orbital will be occupied by the two 2s electrons although different orbitals will be occupied by the three 2p electrons in accordance to Hund's rule.
What is Hund's rule why it is called so?
Answer: Hund's rule state that no pairing occurs until all orbital is singly occupied. Explanation: Hund's rule state that in all the orbital s, p, d or f pairing will not occur until all the subshell of orbital will be singly occupied or every shell contains 1 electron each.
Why is the Hund's rule called the law of maximum plurality?
This is because out of the various possible electronic configurations, only that configuration is correct for which the total spin value is maximum.
What type of configurations violate Hund’s rule?
Every orbital of the same energy must have at least one electron which has identical spin before you deposit two in the same orbital.
Briefly explain the working of Hund’s rule.
As per its first rule, electrons before pairing up always enter an empty orbital. Since electrons are negatively charged particles they repel each...
State Aufbau principle.
It states that in an atom’s ground state the electron enters the lowest energy orbital first and later the higher energy orbitals.
Why is Hund's rule called the rule of maximum multiplicity?
hund's rule is called the rule of maximum multiplicity because out of the various possible electronic configurations, only that configuration is co...
What type of configurations violate Hund’s principle?
Every orbital of the same energy must have at least one electron which has identical spin before you deposit two in the same orbital.
What is stated at the 1st part of the hund’s rule?
The 1st part of the hund’s rule states that, for a particular electronic configuration, the electron having maximum spin multiplicity has the lowes...
What is the nickname of the 1st part of the hund’s rule?
The nickname of the 1st part of the hund’s rule is spin-spin interaction.
Why is hund’s rule not applicable for all elements?
If Hund's rule is not applied the number of singly occupied orbitals or unpaired electrons will decrease gradually. Total number of unpaired electr...
What is the Aufbau principle?
Aufbau principle. This principle explains filling up electrons in rising orbital energy. for example. 1s orbital should be fulfilled before 2s orbital for 1s is lower in energy than 2s orbital. By regarding these three rules, the electron configuration of an atom is composed. for example.
What is the principle of a given electronic configuration?
According to this principle, for a given electronic configuration, the paring of the particle is done after each subshell is filled with a single electron. In other words, the under subshell should have maximum multiplicity. Hund's rule states that:
What is the Hund's rule?
Hund’s rule of maximum multiplicity. The rule states that, for a stated electron configuration, the greatest value of spin multiplicity has the lowest energy term. It says if two or more than two orbitals having the same amount of energy are unoccupied then the electrons will start occupying them individually before they fill them in pairs.
How does Hund's rule work?
Briefly explain the working of Hund’s rule. As per its first rule, electrons before pairing up always enter an empty orbital. Since electrons are negatively charged particles they repel each other . When they occupy their orbitals they can easily minimize rep ulsion.
What is the Hunds rule of maximum multiplicity?
Hunds Rule of Maximum Multiplicity rule states that for a given electron configuration, the term with maximum multiplicity falls lowest in energy. According to this rule electron pairing in p, d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied.
When was the rule of ground state discovered?
This rule was discovered in the year 1925 by Friedrich Hund.
What is the Aufbau principle?
Aufbau principle tells us that the lowest energy orbitals get filled by electrons first. After the lower energy orbitals are filled, the electrons move on to higher energy orbitals. The problem with this rule is that it does not tell about the three 2p orbitals and the order that they will be filled in. According to Hund’s rule:
What Does Hund's Rule Mean?
Hund's rule states that a larger total spin state of an atom sometimes makes the atom more stable. This rule is fairly reliable (with occasional failures) for the determination of the state of a given excited electron configuration. It was discovered in the year 1925 by Friedrich Hund. According to Hund's rule:
Corrosionpedia Explains Hund's Rule
An atom consists of a nucleus around which electrons revolve in orbitals of different energy. According to the Aufbau principle, electrons fill the lowest energy level before filling up the higher ones. Thus, electrons are found in discrete atomic orbitals in an arrangement known as electron configuration.
State Hund's Rule of Maximum Multiplicity Class 11
The Aufbau principle tells us that the lowest energy orbitals will get filled up by electrons first. Thereafter, the electrons move on to energetically higher orbitals. The problem with this rule is that it does not tell about the order in which they will be filled in three 2p orbitals and five 3d orbitals consequently.
Hund's rule states that
1. For a particular electronic configuration, the electron having maximum spin multiplicity has the lowest energy. The multiplicity can be depicted as ( 2S+1), where S represents total spin angular momentum of the electrons.
Spin Multiplicity Meaning
In spectroscopy and in quantum chemistry, the multiplicity of an energy level can be calculated by using 2S+1, where S indicates the total spin angular momentum. States of electrons with multiplicity 1, 2, 3, 4, 5 are respectively called singlets, doublets, triplets, quartets and quintets.
Spin Multiplicity Rule
According to the spin multiplicity rule, for a given electron configuration, the lowest energy term is the one with the greatest value of spin multiplicity. This indicates that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs.
Frequently Asked Question (FAQs) - Hunds Rule - Definition, Examples, Uses, Spin Multiplicity, FAQs
hund's rule is called the rule of maximum multiplicity because out of the various possible electronic configurations, only that configuration is correct for which the total spin value is maximum.