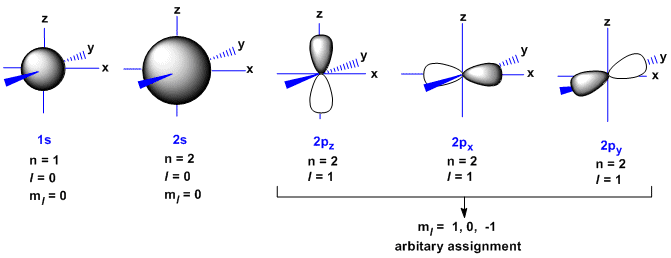
What are the quantum numbers for carbon?
| Name | Symbol | Range of values |
| Principal quantum number | n | 1 ≤ n |
| Azimuthal quantum number (angular moment ... | ℓ | 0 ≤ ℓ ≤ n − 1 |
| Magnetic quantum number (projection of a ... | m ℓ | −ℓ ≤ m ℓ ≤ ℓ |
| Spin quantum number | m s | −s ≤ m s ≤ s |
How do you calculate quantum numbers?
Spin Quantum Number
- Key points on Spin Quantum Number. Quantum numbers give complete information about the electron in an atom.i, e., energy, position, size, shape and orientation of that orbital and the direction ...
- Spin of an Electron. ...
- Example :
- Determination of Magnetic Nature. ...
- Related Videos. ...
What do the quantum numbers actually signify?
What are the quantum numbers?
- Principal quantum number (n). It is the last energy level to fill and indicates the size of the orbital and therefore the distance between the nucleus and the electron. ...
- Azimuthal or secondary quantum number (l). Indicate the shape of the orbital.
- Magnetic quantum number (m). Indicates the orientation of the orbital.
- Spin quantum number (s). ...
How to calculate quantum number?
Vibrational quantum number using vibrational frequency calculator uses Vibrational quantum number = ( Vibrational energy /( [hP] * Vibrational Frequency ))-1/2 to calculate the Vibrational quantum number, The Vibrational quantum number using vibrational frequency formula is defined as a scalar quantum number that defines the energy state of a ...
What are the four quantum numbers?
The ORCA-Quest’s ability to identify the number of photons invites new possibilities for a wide range of fields. For example, the ORCA-Quest accurately observes the quantum state by quantitatively ... illuminated structure, 4.6 µm x 4.6 µm pixel ...
How do you find quantum number of carbon?
Explanation: Carbon (atomic number 6 ) can be found in the second row of the periodic table; at the ground state, the six electrons occupy two of its principle energy levels, giving the electron with the highest energy a principal quantum number n of 2 .
What are the 4 quantum numbers of each element?
In atoms, there are a total of four quantum numbers: the principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number (ms).
What are the quantum numbers of oxygen?
For 8th electron of oxygen atom, the four quantum numbers are n=2,l=1,m=+1 or −1,s=+21 or −21.
What is the quantum number of an element?
These quantum numbers describe the size, shape, and orientation in space of the orbitals on an atom. The principal quantum number (n) describes the size of the orbital. Orbitals for which n = 2 are larger than those for which n = 1, for example.
How do you find quantum number?
Look at the Periodic Table of Elements and find the element that you want to know the quantum number for. Find the principal number, which denotes the element's energy, by looking in which period the element is found. For example, sodium is in the third period of the table, so its principal quantum number is 3.
What are the 4 quantum numbers for hydrogen?
There are four quantum numbers: n, ℓ, mℓ, and ms. Each one is a particular factor in an equation describing a property of the electron. At this introductory level, the equations are not needed.
What is the quantum number of 6th electron of carbon?
Thus, Carbon with electron 6 has four quantum numbers: $2$,$1$,$0$,$ + \dfrac{1}{2}$ . Note: Quantum numbers are important since they can be used to figure out an atom's electron structure and where the atom's electrons are most likely to be found.
What are the 4 quantum numbers for boron?
Z = 5; (Boron): The first four electrons have the same four quantum numbers as Berylium, but the fifth electron is the first one in the l = 1 subshell which can have m(l)-values -1, 0 and +1. For each of these m(l) values, s = + 1/2 and s = - 1/2 spin values also occur...so there are six more possibilities.
What are the quantum numbers for calcium?
Step 5: Final List the quantum numbers for the last valence electron in Calcium: (4, 0, 0, -1/2).
How do you write quantum numbers for electrons?
1:086:23How to Write Quantum Numbers for Electrons - YouTubeYouTubeStart of suggested clipEnd of suggested clipLet's write down the four quantum numbers we know that there is the energy level the sub shell orMoreLet's write down the four quantum numbers we know that there is the energy level the sub shell or the shape. And type of orbital there's the orbital position and then the magnetic spin.
How many quantum numbers are there?
four quantum numbersThe set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers. There are four quantum numbers, namely, principal, azimuthal, magnetic and spin quantum numbers.
How do you read quantum numbers?
0:338:41Quantum Numbers, Atomic Orbitals, and Electron ConfigurationsYouTubeStart of suggested clipEnd of suggested clipRemember these shapes are not electrons just regions in space where electrons can be and each oneMoreRemember these shapes are not electrons just regions in space where electrons can be and each one can hold up to two electrons. The more electrons and atom has the more of these orbitals it will need
What is quantum number?
Updated December 12, 2019. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the Schrödinger wave equation for the hydrogen atom. There are four quantum numbers:
Where are the electrons in a carbon atom?
For the outer valence electrons of a carbon atom, the electrons are found in the 2p orbital. The four quantum numbers used to describe the electrons are n=2, ℓ=1, m=1, 0, or -1, and s=1/2 (the electrons have parallel spins).
Can two electrons have the same quantum number?
According to the Pauli exclusion principle, no two electrons in an atom can have the same set of quantum numbers. Each quantum number is represented by either a half-integer or integer value.
Quantum Numbers of all the elements in the Periodic Table
Refer to graph, table and property element trend below for Quantum Numbers of all the elements in the periodic table. We have shown the Quantum Numbers of the elements for which reliable data is available.
Quantum Numbers Graph - Quantum Numbers of all the elements in graph
Mouseover on the chart to see the element name and Quantum Numbers of the element.
Quantum Numbers Chart - Quantum Numbers of all the elements in table chart
This Quantum Numbers chart table gives the Quantum Numbers of all the elements of periodic table . Click on 'Element Atomic Number', 'Element Symbol', 'Element Name' and 'Element Quantum Numbers' headers to sort.
Periodic Table of Elements with Quantum Numbers Trends
In the below periodic table you can see the trend of Quantum Numbers. For facts, physical properties, chemical properties, structure and atomic properties of the specific element, click on the element symbol in the below periodic table.
How many quantum numbers are there in an atom?
The 4 quantum numbers: In atoms, there are a total of 4 quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number (ms). Principal quantum number (n): The principal quantum number describes the electron shell, or energy level, of an electron.
What is the principal quantum number?
Principal quantum number (n): The principal quantum number describes the electron shell, or energy level, of an electron. The value of n ranges from 1 to the shell of the outermost electron of that atom. The values of n = 1, 2, 3,...
What is the quantum number of electron spin?
The electron spin quantum number can only have 2 values: -1/2 and +1/2. 1/2 describes the magnitude and +/- provides information on the direction of spin. Following Hund's rule, electrons enter unoccupied orbitals of equal energy before completely filling an orbital.
Which orbitals have angular momentum?
Electrons in P-orbitals have an angular momentum quantum number of 1. Electrons in D-orbitals have an angular momentum quantum number of 2. Electrons in F-orbitals have an angular momentum quantum number of 3. The values of l= 0 to (n-1).
How to find the element in the periodic table?
Step 1: Find the element on the periodic table. Step 2: Determine n and l by identifying the period # and the block that the element is located in. Step 3: Determine ml by labeling the block from -l to +l. Step 4: Determine ms by determining if the element is in the first or second half of the block. Step 5: Put all of the information together ...
What is each quantum number?
Each one is a particular factor in an equation describing a property of the electron. At this introductory level, the equations are not needed. The value of each quantum number is assigned to each electron in an atom by a "building up" process.
What is the principle of building up quantum numbers?
Niels Bohr called this process the "Aufbau" principle: aufbau means "building up.". n is ALWAYS the starting point for building up a series of quantum numbers. Each quantum number is then assigned according to a set of rules, each of which took years of study to finally determine.