The two monomers for the synthesis of Nylon 6, 6 are :
- (1) HOOC (CH2) 6COOH, H2N (CH2) 4NH2
- (2) HOOC (CH2) 6COOH, H2N (CH2) 6NH2
- (3) HOOC (CH2) 4COOH, H2N (CH2) 6NH2
- (4) HOOC (CH2) 4COOH, H2N (CH2) 4NH2
What is the monomeric name of nylon 6?
Nylon 6,6 : Nylon 6,6 is comprised of two monomers, Hexamethylenediamine, and adipic acid, each providing six carbon atoms. Hence, the name Nylon 6,6. Also, what type of polymer is nylon 6? It is a semicrystalline polyamide.
What are the two monomers for the synthesis of nylon 6?
The two monomers for the synthesis of Nylon 6, 6are: A HOOC(CH2)6COOH,H2N(CH2)6NH2 B HOOC(CH2)4COOH,H2N(CH2)4NH2 C HOOC(CH2)6COOH,H2N(CH2)4NH2 D HOOC(CH2)4COOH,H2N(CH2)6NH2 Hard JEE Mains Open in App Solution Verified by Toppr Correct option is D)
What is the difference between nylon 6 and 6/6?
On their own, nylon 6/6 is the more sensitive, though nylon 6 is still vulnerable without stabilizers. UV light weakens nylon through interaction with the chemical structure’s pi electrons, specifically double bonds and aromatic groups.
How many carbon atoms are in Nylon 6 6?
The nylon in the pictures on this page is called nylon 6,6, because each repeat unit of the polymer chain has two stretches of carbon atoms, each being six carbon atoms long. Other nylons can have different numbers of carbon atoms in these stretches.
What are the monomers of nylon?
The monomers for nylon 6-6 are adipic acid and hexamethylene diamine. The two molecules are combined to create the polymer and water (H2O) is produced as a by-product.
What polymer is nylon 6 6?
polyamidesNylon (PA) 6 & 66 are both synthetic polymers called polyamides, with the numbers describing the type and quantity of polymer chains in their chemical structure. Most nylons, including 6 & 66, are semi-crystalline and possess good strength and durability for demanding applications.
What is the monomer of nylon 2 nylon-6?
The monomers of Biodegradable polymer, nylon 2-nylon 6 are glycine and amino caproic acid.
What is the monomer of nylon 6 10?
decanedioyl dichlorideNylon-6,10 is made from two monomers, one contains six carbon atoms, the other 10 - hence its name. The 10-carbon monomer is decanedioyl dichloride (ClOC(CH2)8 COCl), an acid chloride with a -COCl group at each end. The other monomer is a six-carbon chain with an amino group, -NH2, at each end.
What is nylon 6 made of?
Nylon 6,6 is prepared by step growth polymerization of hexamethylene diamine and adipic acid. After drying, the nylon 6,6 is melt spun at 280°–290°C into fibers.
What is the monomer of nylon Class 8?
Solution : The monomers of Nylon-66 are : hexamethylene diamine and adipic acid.
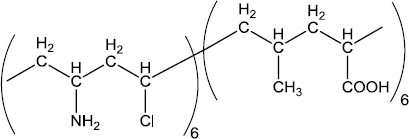
What are the monomer units of the polymer nylon 2 nylon 6 is this polymer biodegradable?
The correct answer of this question is the monomers of biodegradable polymer, nylon 2-nylon 6 are glycine and amino caproic acid.
What is monomer of Buna S?
The monomers of Buna-S rubber are styrene and butadiene.
What is nylon 6 made of?
Nylon 6 is only made from one kind of monomer, a monomer called caprolactam. Caprolactam has 6 carbons, hence 'Nylon 6'. During polymerization, the amide bond within each caprolactam molecule is broken, with the active groups on each side re-forming two new bonds as the monomer becomes part of the polymer backbone.
What does the 6 in nylon mean?
For clarity in pronunciation, the "i" was changed to "y". Nylon 6, in this 6 represents the monomer's number of carbon atoms.
How is nylon 66 synthesized?
Synthesis of Nylon 6,6: Nylon 66 is synthesized by polycondensation of hexamethylenediamine and adipic acid. Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium/carboxylate mixture. The nylon salt goes into a reaction vessel where polymerization ...
What is nylon polymer?
Nylon - the first synthetic polymer whose physical properties superior to the properties of some metals. Nylon has an incredible combination of properties - high strength, medium stiffness and resistance to high temperature (+85. Continue Reading.
Why is nylon 6.6 called nylon?
The name of this fiber appeared in 1939 at the world fair in New York - "NYlon" ("nylon" - the first letters of the city name New York.has the name "nylon 6.6", because each repeating unit of the polymer chain contains two sites of carbon atoms, each of which contains six carbon atoms.
Which group of nylon is capable of forming hydrogen bonds?
Image from - The Polymer Science Learning Center. When the chains of Nylon 6 and Nylon66 are visualized, the -NH group and -CO- group in the back bone is capable of forming hydrogen bond.
Which is better nylon 66 or 66?
Nylon 66 is better nylon with more crystalline nature ,higher melting point,better acid resistance,better stiffness and better tensile modulus etc.Nylon 66 should be used if a high performing engineering plastic is required that will be exposed to higher temperatures.
Answer
First of all, nylon 6 is only made from one kind of monomer, a monomer called caprolactam. Nylon 6,6 is made from two monomers, adipoyl chloride and hexamethylene diamine
New questions in Chemistry
One of those responsible for the evil announced through cigarettes is tar, which corresponds to a mixture of aromatic substances, including naphthalen …
How many carbon atoms are in nylon 6?
Nylon 6 is derived from one monomer, which is a molecule that can be bonded to other identical molecules to form polymers. For nylon 6, the monomer has six carbon atoms, hence the name nylon 6. Nylon 6/6 is made from two monomers. Each of these monomers has six carbon atoms, which is reflected in the name nylon 6/6.
Why is nylon 6 more absorbent than 6?
The reason: nylon absorbs moisture from the air. Failure to dry the material will lead to splays and marks on part surfaces.
What is nylon used for?
Nylon is used to make everything from barbed snap rivets and push rivets to cable twist ties, door panel retaining clips and cable strain reliefs. The material is a polyamide with many variants, but the most common we see used in engineering applications are nylon 6 and nylon 6/6, also referred to as nylon 66 and nylon 6.6, ...
How much glass fibers to add to nylon 6?
By adding 30% glass fibers to nylon 6, this can be reduced to 0.4%. Add 33% to nylon 6/6, and you’re looking at 0.5%. All that aside, when you need to enhance the strength of nylon, glass filled, or GF nylon, is ideal. In fact, by using glass as an additive, you can strengthen your nylon by up to 70% more than untreated nylon.
Can nylon 6 shrink?
When nylon 6/6 is exposed to ambient air temperatures and begins to solidify, mold shrinkage occurs and shapes can change. However, you can get around this by increasing the dimensions of your extrusion dies and injection molds. Nylon 6 is another matter, which processes much easier.
Is nylon 6 UV resistant?
Nylons are not UV resistant, although you can add stabilizers to give them almost any effect you desire. On their own, nylon 6/6 is the more sensitive, though nylon 6 is still vulnerable without stabilizers.
Can nylon be used outdoors?
Let’s be fair, however. UV rays affect all materials, not just nylons. But with stabilizers, nylon can perform extremely well outdoors. For example, these mini snap together rivets made of nylon 6/6 with a UL94 V-2 flammability rating are ideal for outdoor applications: