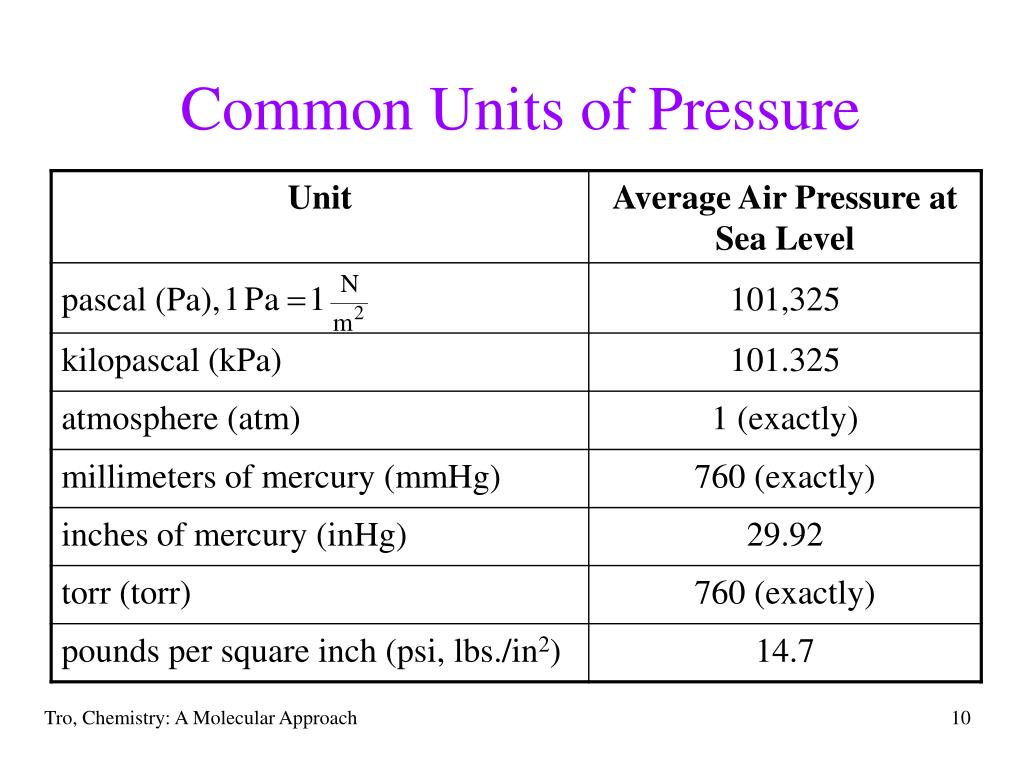
- Pascals (Pa) The standard SI unit of pressure is Pa, named after scientist Blaise Pascal. The atmospheric pressure in pascals is 101,325 Pa, or about 100 = kPa.
- Standard Atmospheres (atm) The unit atmosphere (atm) is convenient because it is about equal to the atmospheric pressure. ...
- torr (Torr) The unit torr is almost equal to 1 mm of Hg, which you can read about in the section of manometric units.
- bar (bar) The unit bar is super pointless. The bar is a metric unit that is the same as 100,000 Pa, or 100 kPa, or 0.1 MPa.
- Pounds per Square Inch (psi or lb/in 2) American units (yes I’m American, it’s called self-deprecating humor). Pounds per square inch means the pound-force per square inch.
- Technical Atmosphere (at or kgf/cm 2) The technical atmosphere (at) is the metric equivalent of psi, and is measured in kilogram-force per square centimeter.
- Barad (Ba) The barad (Ba) is a cgs unit that is so unstandardized, it has many names. ...
- Pièze (pz) If you see this unit, you’re in trouble. The pièze is the standard unit of pressure in the mts measurement system which was used in the Soviet Union ...
What units of pressure are commonly used in the US?
The United Kingdom:
- 1862 – preparations for conversion to the metric system began, and metric units could be legally used in the UK for nearly a century before efforts to fully convert to ...
- 1965 – the government set a 10-year plan for full metrication.
- 1969 – the Metrication Board was created to promote and coordinate country-wide metrication. ...
What units are most often used to describe pressure?
The units of pressure are generally expressed as P = F/A. The examples include dynes per square inch, pounds per square inch, and Newtons per square metre. The pressure can be defined as force per unit area that is perpendicular to the surface. The formula for this can be expressed like P = F/A.
What are the units used for pressure?
what units are used to express pressure measurements
- Gas Pressure Unit Conversions – torr to atm, psi to atm, atm to mm Hg, kpa to mm Hg, psi to torr
- Definitions of Absolute Pressure, Gauge Pressure, Atmospheric Pressure and Vacuum Pressure
- Pressure Measurement Manometers
What unit is used to measure standard pressure?
- Pascal (Pa)
- Atmospheric pressure (atm)
- Pound per square inch (psi)
- 760 mm of Hg/ torr
What are the units of pressure?
Other Units Of Pressure. There are several other units of pressure such as pounds per square inch and bar, unit of at mospheric pressure is atm, centimetres of water, millimetres of mercury or inches of mercury (used as a unit of blood pressure), torr, MSW and FSW.
What is the formula for pressure?
The pressure is a physical quantity and mostly expressed as formula p = F/A where F and A are force per area perpendicular to the surface. While we have already discussed everything about pressure in our earlier article, here we will explore the topic of units of pressure.
What is SI unit?
This is a modern version of the metric system and is popularly used when measuring physical quantities.
common units of pressure Definition
Pressure is a physical quantity which is defined as force per unit area. Force has been specified in terms of mass and acceleration, which has many different units. So, pressure has several units due to combination of unit of mass and area. Some common units are Pascal, bar, atm, mmHg and torr.
Overview of Common Units Of Pressure
Pressure is one of the important physical quantities which have been used in several fields of engineering, physics, chemistry and medical science. Pressure in basic sense can be defined as amount of the force which is acting perpendicularly on the surface per unit area. It is denoted by the symbol ‘P’.
Pressure
Sometimes it’s necessary to not only know the magnitude of force, but the surface area where the force has been distributed is also important. So, in those applications where the area defined the amount of force, pressure is used.
SI and CGS units of pressure
SI unit system and CGS unit system are the International standard unit system assigned for any physical quantities.
Other common units of pressure
Apart from SI and CGS units, pressure has other common units which are frequently used in several applications.
The most frequently used pressure units
The most frequently used pressure units are pascal (Pa), kilopascal (kPa), megapascal (MPa), psi (pound per square inch), torr (mmHg), atm (atmospheric pressure), and bar.
Pascal Conversion
Pascal is a derived metric pressure unit and equals to 1 newton per square meter.
Kilopascal (kPa) Conversion
Kilopascal (kPa) is a frequently used pressure unit and equals to 1000 newton per square meter (metre).
Bar Conversion
Bar is a metric pressure unit and equals to 100 kilopascals which is almost equal to the atmospheric pressure.
Psi (Pound Force Per Square Inch) Conversion
Psi is a pressure unit and equals to the force of one pound applied to one square inch.
Atm (Atmospheric Pressure) Conversion
An atmosphere (atm) equals to the air pressure at the sea level at a temperature of 15 Celsius.
Inch of Mercury Conversion
Inch of Mercury (inHg) is the pressure exerted by a column of mercury 1 inch (25.4 mm) in height.
What are the Common Units of Pressure?
Pressure is something that we experience in daily life but fail to recognize it as our body is so accustomed to it. For example, the atmospheric air applies pressure on our body, but we don’t feel it as we are exposed to it since birth and our body is habitual to it.
Imperial Units of Pressure
Different countries use units which are local to their use only. For example, the USA uses different units of mass and area other than the SI unit system. Hence it creates a different set of units of pressure. Mass is commonly known as pound or ounce and the distance is measured in inches or feet.
CGS Unit of Pressure
Centimeter-gram-second (CGS) unit system uses, centimeter for distance and a gram for measuring mass. It is a very old system of measurement. In this pressure is measured in barye, which is defined as below
Atmospheric Units
When one wants to calculate atmospheric pressure, unit standard atmospheric pressure is used, written as atm. This atm is equal to 101325 Pascal.
Pressure in Terms of Height of Liquid Column
In order to measure pressure in pipes in industries, earlier U-tube manometer were used. They are nothing but a transparent tube with U shape and markings on them, with a high-density liquid filled in it, the difference in height of the liquid column is used to measure the pressure in the tube.
Context and Applications
This topic is significant in the professional exams for both undergraduate and graduate courses, especially for