Apart from these elements, the following elements are also known to be classified as metalloids in some circumstances:
- Carbon
- Aluminium
- Selenium
- Polonium
- Astatin
Which metalloids would behave more like metals?
The Marvelous Metalloids of the Periodic Table
- Characteristic Properties of Metalloids. Metalloids can conduct electricity, but not as well as metals. ...
- A List of Metalloids. The element boron has a strong tendency to form covalent bonds, meaning that chemically, it is a nonmetal, like carbon or silicon.
- Conclusion on Metalloids. ...
Which lists only metalloids?
Which lists only metalloids? boron (B), germanium (Ge), and tellurium (Te) Which element is a metalloid? Check all that apply. polonium (Po) silicon (Si) antimony (Sb) The metalloid that has three valence electrons is . boron. The metalloid that has five valence electrons in the fourth electron shell is .
What are 3 examples of metals?
Metals:
- Lithium
- Beryllium
- Sodium
- Magnesium
- Aluminium
- Potassium
- Calcium
- Scandium
- Titanium
- Vanadium (Rest you can see in the pic)
How many elements are metalloids?
There are six elements generally accepted to be metalloids. However, based on the classification criteria being used, the exact number may vary, ranging from six to nine elements. What are 4 properties of metalloids?
What are the 10 examples of metalloids?
Following are the elements that are considered to be metalloids:Boron (B)Silicon (Si)Germanium (Ge)Arsenic (As)Antimony (Sb)Tellurium (Te)Polonium (Po)
What are metalloids 2 examples?
Examples of metalloids include boron (B), silicon (Si), germenium (Ge), arsenic (As) and antimony (Sb).
What are metalloids 9 examples?
Metalloids are elements which have properties intermediate between those of metals and non-metals. Examples are silicon, germanium etc.
What are metalloids 4 examples?
Boron, silicon, germanium, arsenic, antimony, tellurium, and polonium are metalloids.
What are metalloids class8?
Metalloids are elements that show physical and chemical properties of metals and non metals both. Elements like boron, silicon, germanium, arsenic, antimony, tellurium are recognized as metalloids.
What are metalloids short answer?
1 : an element intermediate in properties between the typical metals and nonmetals. 2 : a nonmetal that can combine with a metal to form an alloy.
What are metalloids?
Elements regarded as metalloids. The elements commonly classified as metalloids are boron, silicon, germanium, arsenic, antimony and tellurium. The status of polonium and astatine is not settled.
What are the elements that are considered metalloids?
One or more of carbon, aluminium, phosphorus, selenium, tin or bismuth, these being periodic table neighbours of the elements commonly classified as metalloids, are sometimes recognised as metalloids.
Is Selenium a metalloid?
Selenium, in particular, is commonly designated as a metalloid in environmental chemistry on account of similarities in its aquatic chemistry with that of arsenic and antimony. There are fewer references to beryllium, in spite of its periodic table position adjoining the dividing line between metals and nonmetals.
What is a metalloid?
v. t. e. A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids.
What are the elements that are classified as metalloids?
Only the elements at or near the margins, lacking a sufficiently clear preponderance of either metallic or nonmetallic properties, are classified as metalloids.
What are the two catalysts used in organic synthesis?
Boron trifluoride and trichloride are used as catalysts in organic synthesis and electronics; the tribromide is used in the manufacture of diborane. Non-toxic boron ligands could replace toxic phosphorus ligands in some transition metal catalysts. Silica sulfuric acid (SiO 2 OSO 3 H) is used in organic reactions. Germanium dioxide is sometimes used as a catalyst in the production of PET plastic for containers; cheaper antimony compounds, such as the trioxide or triacetate, are more commonly employed for the same purpose despite concerns about antimony contamination of food and drinks. Arsenic trioxide has been used in the production of natural gas, to boost the removal of carbon dioxide, as have selenous acid and tellurous acid. Selenium acts as a catalyst in some microorganisms. Tellurium, its dioxide, and its tetrachloride are strong catalysts for air oxidation of carbon above 500 °C. Graphite oxide can be used as a catalyst in the synthesis of imines and their derivatives. Activated carbon and alumina have been used as catalysts for the removal of sulfur contaminants from natural gas. Titanium doped aluminium has been identified as a substitute for expensive noble metal catalysts used in the production of industrial chemicals.
How are metalloids obtained?
The recognised metalloids can be obtained by chemical reduction of either their oxides or their sulfides. Simpler or more complex extraction methods may be employed depending on the starting form and economic factors. Boron is routinely obtained by reducing the trioxide with magnesium: B 2 O 3 + 3 Mg → 2 B + 3MgO; after secondary processing the resulting brown powder has a purity of up to 97%. Boron of higher purity (> 99%) is prepared by heating volatile boron compounds, such as BCl 3 or BBr 3, either in a hydrogen atmosphere (2 BX 3 + 3 H 2 → 2 B + 6 HX) or to the point of thermal decomposition. Silicon and germanium are obtained from their oxides by heating the oxide with carbon or hydrogen: SiO 2 + C → Si + CO 2; GeO 2 + 2 H 2 → Ge + 2 H 2 O. Arsenic is isolated from its pyrite (FeAsS) or arsenical pyrite (FeAs 2) by heating; alternatively, it can be obtained from its oxide by reduction with carbon: 2 As 2 O 3 + 3 C → 2 As + 3 CO 2. Antimony is derived from its sulfide by reduction with iron: Sb 2 S 3 → 2 Sb + 3 FeS. Tellurium is prepared from its oxide by dissolving it in aqueous NaOH, yielding tellurite, then by electrolytic reduction: TeO 2 + 2 NaOH → Na 2 TeO 3 + H 2 O; Na 2 TeO 3 + H 2 O → Te + 2 NaOH + O 2. Another option is reduction of the oxide by roasting with carbon: TeO 2 + C → Te + CO 2.
What is a period of metalloids?
Periods (1–7, ...) Blocks (s, p, d, f, ...) A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids.
Where are metaloids located?
Metalloids lie on either side of the dividing line between metals and nonmetals. This can be found, in varying configurations, on some periodic tables. Elements to the lower left of the line generally display increasing metallic behaviour; elements to the upper right display increasing nonmetallic behaviour. When presented as a regular stairstep, elements with the highest critical temperature for their groups (Li, Be, Al, Ge, Sb, Po) lie just below the line.
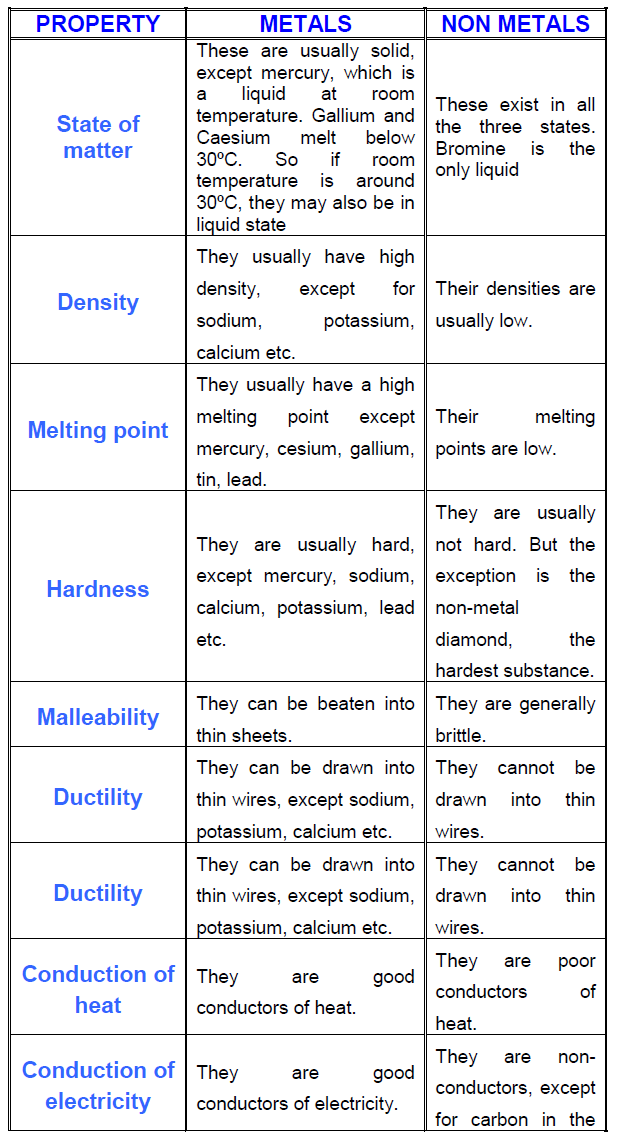
What are the properties of metals?
The properties of form, appearance, and behaviour when mixed with metals are more like metals. Elasticity and general chemical behaviour are more like nonmetals. Electrical conductivity, band structure, ionization energy, electronegativity, and oxides are intermediate between the two.
What is a metalloid?
What is the definition of the term ‘metalloid’? A metalloid is a chemical element that exhibits some metal and some nonmetal properties. Metalloids form a jagged region in the periodic table separating elements which have clear metallic properties from elements which have clear non-metallic properties.
How to determine if an element is metalloid?
The easiest way to decide if a given unknown element is a metalloids is by testing whether any metal and non-metal characteristics can be detected . If both are detected, then the given element is most likely to be a metalloid element.
What are the elements that are found in the step-like line between metals and nonmetals?
They are: antimony (Sb), germanium (Ge), silicon (Si), arsenic (As), tellurium (Te), polonium (Po), boron (B), and astatine (At).
What element can form alloys with MnB?
In fact, ferroboron (which contains 15 per cent boron ) is widely used in order to inject boron into steel.
What are nickel boron alloys used for?
Furthermore, nickel-boron alloys are used as ingredients in the engineering industry for welding alloys and case hardening compositions. Silicon alloys of aluminium and iron are widely used in the construction and automotive industries.
What is the dividing line between metals and nonmetals?
Some periodic tables have a dividing line between metals and nonmetals, and below this line, the metalloids can be found. Typically, metalloids have metallic appearances but they are usually brittle and only mediocre electricity conductors. Chemically, these elements usually behave as non-metals. Metalloids have the ability to form metallic alloys.
What is boron used for?
Furthermore, boron is used in herbicides and also in insecticides and. This element is an active trace element, which has several antiseptic, antiviral, and antifungal properties in the form of boric acid.
What are some examples of metalloids?
The elements classified as metalloids are - boron, silicon, germanium, arsenic, antimony, and tellurium (and sometimes bismuth, polonium, and astatine ).
What are the elements that are considered metalloids?
The six elements that are unanimously considered to be metalloids are the following: Boron. Silicon. Germanium. Arsenic. Antimony. Tellurium. Apart from these six elements, the definition of metalloid elements sometimes includes the elements bismuth, polonium, and astatine as well.
What are metalloids used for?
Used in the creation of certain alloys, cast iron, and ceramics. Lesson Summary. Metalloid elements are elements with properties of both metals and nonmetals. The elements classified as metalloids are - boron, silicon, germanium, arsenic, antimony, and tellurium (and sometimes bismuth, polonium, and astatine ).
What is a metalloid element?
Metalloid elements, also known as semimetals, are elements that have properties of both metals and nonmetals. The metalloid definition is considered to include between six to nine elements that occur along a slanted line between the metal and nonmetal elements of the periodic table. The six elements that are unanimously considered ...
How many types of metalloids are there?
There are three distinct categories of metalloid elements based on the number of valence electrons, and the chemical properties within each category are fairly similar.
Why are metalloids ambiguous?
This ambiguity is in large part due to a lack of specific properties that are considered characteristics of all metalloids. Instead, the metalloid elements are simply characterized as having a mix of properties that are in between the properties of metals and nonmetals.
Where are metalloids located?
The metalloids are located along a slanted line between the metal elements and nonmetal elements of the periodic table. They span from Group 13 to Group 16, 17, or 18 based on what criteria of classifying metalloid elements is being used.
Overview
Nomenclature and history
The word metalloid comes from the Latin metallum ("metal") and the Greek oeides ("resembling in form or appearance"). Several names are sometimes used synonymously although some of these have other meanings that are not necessarily interchangeable: amphoteric element, boundary element, half-metal, half-way element, near metal, meta-metal, semiconductor, semimetal and submetal. "Amphoteric element" is sometimes used more broadly to include transition metals ca…
Definitions
A metalloid is an element that possesses a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals, and which is therefore hard to classify as either a metal or a nonmetal. This is a generic definition that draws on metalloid attributes consistently cited in the literature. Difficulty of categorisation is a key attribute. Most elements have a mixture of metallic and nonmetallic properties, and can be classified according to which set of propertie…
Periodic table territory
Metalloids lie on either side of the dividing line between metals and nonmetals. This can be found, in varying configurations, on some periodic tables. Elements to the lower left of the line generally display increasing metallic behaviour; elements to the upper right display increasing nonmetallic behaviour. When presented as a regular stairstep, elements with the highest critical temperature for their groups (Li, Be, Al, Ge, Sb, Po) lie just below the line.
Properties
Metalloids usually look like metals but behave largely like nonmetals. Physically, they are shiny, brittle solids with intermediate to relatively good electrical conductivity and the electronic band structure of a semimetal or semiconductor. Chemically, they mostly behave as (weak) nonmetals, have intermediate ionization energies and electronegativity values, and amphoteric or weakly acidic oxides. They can form alloys with metals. Most of their other physical and chemical prop…
Common applications
The focus of this section is on the recognised metalloids. Elements less often recognised as metalloids are ordinarily classified as either metals or nonmetals; some of these are included here for comparative purposes.
Metalloids are too brittle to have any structural uses in their pure forms. They and their compounds are used as (or in) alloying components, biological agent…
Elements commonly recognised as metalloids
Properties noted in this section refer to the elements in their most thermodynamically stable forms under ambient conditions.
Pure boron is a shiny, silver-grey crystalline solid. It is less dense than aluminium (2.34 vs. 2.70 g/cm ), and is hard and brittle. It is barely reactive under normal conditions, except for attack by fluorine, and has a melting point …
Elements less commonly recognised as metalloids
Carbon is ordinarily classified as a nonmetal but has some metallic properties and is occasionally classified as a metalloid. Hexagonal graphitic carbon (graphite) is the most thermodynamically stable allotrope of carbon under ambient conditions. It has a lustrous appearance and is a fairly good electrical conductor. Graphite has a layered structure. Each layer consists of carbon ato…