Factors Affecting Buffer Capacity
- Ratio of [A– ]/ [HA]. The buffer capacity depends essentially on the ratio of the salt to the acid or base. The...
- Total Buffer Concentration:. Buffer capacity depends upon the total buffer concentration. For example, it will take...
- Temperature:. Buffers are commercially available with a wide range of pH values, and they come...
What is the effect of temperature on buffer capacity?
The other assumption is that temperature has no effect on buffering capacity since buffering capacity apparently only depends on two factors: concentration of the buffer solution and the ratio of pH to pKa.
Why does buffer capacity decrease with age?
Buffer capacity is reduced both in baby skin and in aged skin. External factors, water, and detergent may reduce the local buffer capacity because of the elution of buffer chemicals leading to increased pH and irritative contact dermatitis.
What is the meaning of buffer capacity?
Buffer capacity is also known as buffer efficiency, buffer value, buffer coefficient, and buffer index. Buffer capacity of a solution is determined the magnitude of resistance to a pH in adding to an acid or base.
How do you increase the capacity of buffer?
It is increased by increasing the concentration of the buffer system components. By doubling the total molar concentration of the buffer system will double the capacity of buffer at a given pH.
What factors influence buffer capacity?
Factors Affecting Buffer CapacityRatio of [A– ]/[HA] The buffer capacity depends essentially on the ratio of the salt to the acid or base. ... Total Buffer Concentration: Buffer capacity depends upon the total buffer concentration. ... Temperature: ... Ionic Strength:
What controls buffer capacity?
The pH change will increase (or decrease) more drastically as the buffer is depleted: it becomes less resistant to change. Buffer capacity is determined through a titration, a technique in which a known volume and concentration of a base or acid is added to the analyte of unknown concentration (Figure 2).
What would increase the buffer capacity of a substance?
The more concentrated the buffer solution, the greater its buffer capacity. As illustrated in Figure 1, when NaOH is added to solutions that contain different concentrations of an acetic acid/sodium acetate buffer, the observed change in the pH of the buffer is inversely proportional to the concentration of the buffer.
What is buffer capacity and what determines its capacity?
Buffer capacity (β) is defined as the amount of a strong acid or a strong base that has to be added to 1 liter of a buffer to cause a pH change of 1.0 pH unit: The buffer capacity depends on the amounts of substance of the weak acid and its conjugated base in the buffer.
Does temperature affect buffer capacity?
The slops and position of pH: log Pco, buffer lines are altered by changes in temperature. At reduced temperatures the buffering capacity is increased, so that the addition of acid or alkali gave rise to smaller changes in pH.
Does volume affect buffer?
Firstly, if the volume changed drastically, i.e. large volumes of water was added to the buffer, the pH will tend to move towards 7. Which is the pH of water. This is because concentration of H+ ions tends to be closer to the amount from auto-ionization of water.
Does adding water affect buffer capacity?
0:063:19What is the Effect of Adding Water on the pH of a Buffer? - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe short answer is that there's no effect and the ph is constant as long as you're not adding.MoreThe short answer is that there's no effect and the ph is constant as long as you're not adding.
What is a simple way to increase the buffer capacity in a solution?
Ways to make a bufferAdding a conjugate base to a weak acid.Adding a strong acid to a weak base.Adding a strong base to a weak acid.
What makes a buffer effective?
Buffer solutions are most effective at resisting a change in pH in either direction when the concentration of the weak acid is equal to the concentration of the conjugate base. And when the concentrations are equal to each other, the ratio is equal to one, and the log of one is equal to zero.
What is the influence of concentration on buffer capacity?
A higher buffer concentration has a greater buffer capacity. This means that a greater amount of hydrogen ions, or a stronger acid, would have to be added to disrupt the equilibrium and change the pH of the buffer. Buffer capacity is also affected by the relative concentrations of the buffer components.
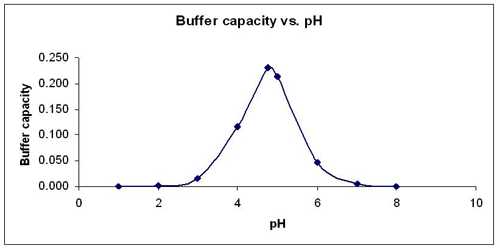
Why buffer solutions exhibit maximum buffering capacity at a particular pH?
At this pH, you have equal concentrations of the acidic and basic components of the buffer systems so that it will be easier for this solution to resist drastic changes in pH upon addition of small amounts of acids and alkali.
What does a lower buffer capacity mean?
Buffer capacity is the amount of acid that buffers are capable of absorbing prior to breaking the capacity for adding strong acid. Solutions with a weaker base have more buffer capacity when adding a strong acid. Solutions with higher amounts of weak acid have higher levels of buffer capacity when adding a strong base.
2. Total Buffer Concentration
Buffer capacity depends upon the total buffer concentration. For example, it will take more acid or base to deplete a 0.5 M buffer than a 0.05 M buffer. The relationship between buffer capacity and buffer concentrations is given by the Van Slyke equation:
3. Temperature
Buffers are commercially available with a wide range of pH values, and they come in both premixed liquid form or as convenient dry powder, capsules, or tablets (to be added to distilled water). These solutions contain acids and bases whose equilibrium is dependent on temperature. Thus, the precise pH is also a function of temperature.
4. Ionic Strength
Ionic strength is reduced by dilution. Change in ionic strength changes the pH of buffer solution resulting in decreased buffer capacity. So, whenever the pH of buffer solution is mentioned ionic strength should be specified.
Why is buffer capacity important?
Buffer capacity of river water is very important, usually necessitating narrow pH ranges that are critical to the survival of most organisms. If the buffer capacity of river water is too small or the pH of the water is outside its buffer range, it can be lethal to the river’s ecosystem. According to Van Vooren, ...
Why does a buffer resist pH changes?
A buffer resists changes in pH due to the addition of an acid or base though consumption of the buffer. As long as the buffer has not been completely reacted, the pH will not change drastically. The pH change will increase (or decrease) more drastically as the buffer is depleted: it becomes less resistant to change.
What happens when acid is added to a buffer?
When an acid or base is added to a buffer system, the effect on pH change can be large or small, depending on both the initial pH and the capacity of the buffer to resist change in pH.
Saturday, March 2, 2019
Buffer capacity is also known as buffer efficiency, buffer value, buffer coefficient, and buffer index. Buffer capacity of a solution is determined the magnitude of resistance to a pH in adding to an acid or base. Buffer capacity relies on the concentration of the buffer components.
Factors affecting buffer capacity
Buffer capacity is also known as buffer efficiency, buffer value, buffer coefficient, and buffer index. Buffer capacity of a solution is determined the magnitude of resistance to a pH in adding to an acid or base. Buffer capacity relies on the concentration of the buffer components.
What is the buffer capacity of skin?
The skin possesses a fairly high buffer capacity, which is determined by the amount of H+ or OH- ions that is needed until the pH value of a solution changes by the unit 1. Buffers contain a weak or medium strong acid (base) and the corresponding salt.
What is a buffer system?
A buffer system is a solution that resists a change in pH when acids or bases are added. The skin possesses a fairly high …. Each biological system possesses a widely unrecognized buffer system to maintain acid-base balance to a specific pH. Our lives are dependent on the functioning of buffer systems. A buffer system is a solution that resists ...
Why is the buffer capacity reduced in baby skin?
External factors, water, and detergent may reduce the local buffer capacity because of the elution of buffer chemicals leading to increased pH and irritative contact dermatitis.
Organic Chemistry Podcast?
I was wondering if there would be interest in having a podcast for organic chemistry. I would use the audio from my youtube videos and putting them into podcast format. I know it'll be harder to portray my message for topics down the road in terms of reactions but just wanted to gauge interest.
would this work by first reducing with LiAlH4 and then treating with a base?
Post your questions about chemistry, whether they're school related or just out of general interest. Please do not post entire problem sets or questions that you haven't attempted to answer yourself. Also please don't use this sub to cheat on your exams!! This is apparently a thing now that people are writing exams from home.
Why does buffering capacity decrease when temperature increases?
Some said that the buffering capacity decreases when temperature increases because of the increase in ionisation of the weak acid molecules and therefore, some of the conjugate base ion will be used to neutralised the H X + ion from the ionization of the weak acid molecules.
Does temperature affect buffering capacity?
The other assumption is that temperature has no effect on buffering capacity since buff ering capacity apparently only depends on two factors: concentration of the buffer solution and the ratio of pH to pKa. So even if the pKa changes due to the change in temperature, the pH also changes accordingly whereby pH increases when pkA increases ...