What is the difference between physical change and sublimation?
In a physical change NO new substances are formed. After sublimation a solid changes to a gas, but it is still the same substance. Only the state of matter has changed.
What are the two types of changes in matter?
Changes can be either physical or chemical. The term ‘matter’ refers to anything that has mass and occupies space. It is made up of tiny particles and has physical and chemical properties. Physical properties of matter include its appearance and observable properties.
What happens to the composition of matter during a physical change?
During a physical change, neither the composition nor the chemical nature of matter is changed. During this change, molecules rearrange themselves without affecting the internal composition. A physical change doesn’t affect the chemical property.
What is a chemical property?
A chemical property is a property exhibited during a chemical reaction. This includes pH, reactivity, inflammability, etc. Let’s learn about Physical and Chemical Changes, how they are related to physical and chemical properties.
Is sublimation a physical or chemical change *?
Can sublimation be a chemical change?
What change property is sublimation?
Why sublimation is a physical change?
What type of reaction is sublimation?
Is magnetizing steel a chemical change chemical property physical change or physical property?
Is sublimation of iodine a chemical change?
Is the sublimation of dry ice a chemical change?
What happens in sublimation?
Why is sublimation considered a physical change and not a chemical change?
Is transpiration chemical change?
Is sublimation endothermic or exothermic?
| Phase Change | Phase to Phase Ex. Solid to Gas | Endothermic or Exothermic? |
|---|---|---|
| Freezing | Liquid to Solid | Exothermic |
| Vaporization | Liquid to Gas | Endothermic |
| Condensation | Gas to Liquid | Exothermic |
| Sublimation | Solid to Gas | Endothermic |
What is a Chemical Change?
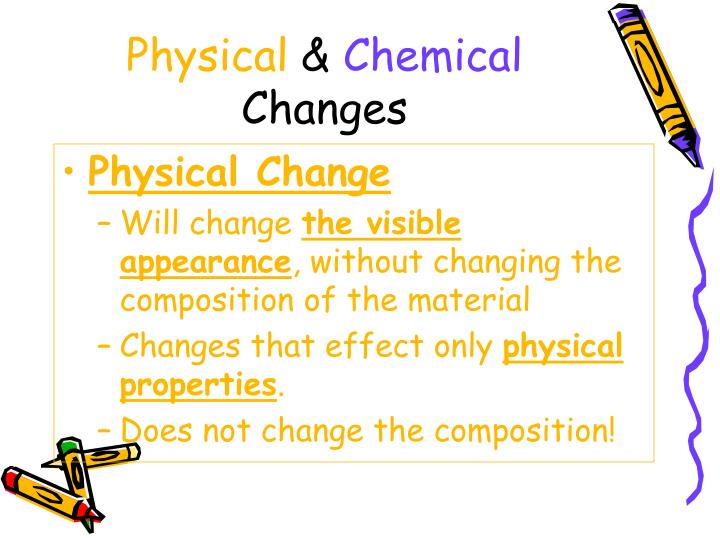
Matter can go through physical changes and chemical changes. A physical change occurs when a substance changes states of matter, or its size or shape. During a physical change the substance, element, or compound does not change. However, the change might involve changes in energy.
What Are Some Examples of Chemical Changes?
Cellular respiration is a chemical reaction in which glucose molecules break down in the presence of oxygen and form carbon dioxide and water. Because glucose is a more complex molecule that breaks down and forms simpler carbon dioxide and water molecules, cellular respiration is considered a decomposition reaction.
What happens to matter during a physical change?
The appearance or shape of matter changes during a physical change, but the kind of matter in the material does not. A chemical change, on the other hand, results in the formation of at least one new substance with new characteristics.
What is chemical property?
A chemical property is a property exhibited during a chemical reaction. This includes pH, reactivity, inflammability, etc. Let’s learn about Physical and Chemical Changes, how they are related to physical and chemical properties.
What is chemical reaction?
It is commonly called a chemical reaction. Different substances have different chemical property. According to this property, substances show variation in their reactivity. A chemical reaction results in a new product. During a chemical change, bonds between the molecules break and the composition of the substance change.
How to tell if a chemical reaction is taking place?
Another sign that a chemical reaction is taking place is a change in hue… . This hue shift is the result of a chemical reaction. However, one must exercise caution since occasionally a colour shift is just the result of combining two hues rather than a true change in the composition of the substances in issue.
What is the term for anything that has mass and occupies space?
Everything around us undergoes certain changes. Changes can be either physical or chemical. The term ‘matter’ refers to anything that has mass and occupies space. It is made up of tiny particles and has physical and chemical properties.
What are some examples of physical changes?
Changes in the size or form of matter are examples of physical change. Physical changes include transitions from one state to another, such as from solid to liquid or liquid to gas. Cutting, bending, dissolving, freezing, boiling, and melting are some of the processes that create physical changes.
Does physical change affect chemical properties?
During this change, molecules rearrange themselves without affecting the internal composition. A physical change doesn’t affect the chemical property.
What Are Physical Properties?
Table of Contents
Intensive and Extensive Properties
- There are two types of physical properties: intensive properties and extensive properties. Intensive properties: An intensive property is a bulk property, which means that it is a physical property of a system that is independent of system size or material content. Temperature, refractive index, density, and hardness of an object are examples of intensive properties. For exa…
Change in Physical Properties Leads to Physical Change
- A physical change occurs without any changes in molecular composition. The same element or compound is present both before and after the change. Throughout the changes, the same molecule is present. Because some measurements necessitate changes, physical changes are linked to physical properties. Some examples of physical changes are- 1. Changes in...
Examples of Physical Properties
- Some examples of physical properties are: 1. Area – the measurement of a two-dimensional surface in a plane. 2. Boiling point – the temperature at which a liquid vaporises. 3. Colour – the quality of an object or substance in terms of light reflected by the object, as measured visually by the hue, saturation, and brightness of the reflected light. 4. Density – a substance’s mass per uni…
How to Measure Physical Properties?
- All physical quantities must be measured. The value of a physical quantity can be expressed as the product of its numerical value and the unit in which it is expressed. The concept of significant figures is used to express the number’s accuracy. S.I. units are used to express measurement units.
Difference Between Physical and Chemical Properties
- Matter refers to everything we see and touch around us. Every matter has unique properties and characteristics that aid in classification and identification. The primary distinction between Physical and Chemical Properties is that when a chemical reaction occurs on a substance, its molecular structure changes. Whereas a substance’s physical properties can be identified as its …