...
Octahedral.
Is an octahedral molecule non-polar?
The molecule is non-polar since it is a symmetrical shape. Here are some examples of Octahedral-shaped molecules: What contributes to this shape?
What is an octahedral shape?
The Octahedral shape is a type of shape which a molecule takes form of when there are 6 bonds attached to a central atom with 4 on the same plane. There are no lone pairs attached to it.
What are the alternatives to octahedral geometry?
For compounds with the formula MX 6, the chief alternative to octahedral geometry is a trigonal prismatic geometry, which has symmetry D 3h. In this geometry, the six ligands are also equivalent. There are also distorted trigonal prisms, with C 3v symmetry; a prominent example is W (CH 3) 6.
What group does an octahedron belong to?
The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group O h.
Is octahedral square planar polar or NonPolar?
NonPolarThe shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom gives the molecule a square planar shape....Square Planar.Shape:square planarPolar/NonPolar:NonPolarHybridization:sp3d2Example:XeF42 more rows•Aug 21, 2020
Is tetrahedral polar or NonPolar?
Tetrahedral is polar depending on geometry and can also be nonpolar. The three factors governing polarity are shape, electronegativity and dipole moment .
Is trigonal pyramidal polar or NonPolar?
polarHence, a trigonal planar molecule (BF3) is nonpolar because the bond polarities cancel each other, but a trigonal pyramidal molecule (NH3) is polar.
Are Bent structures polar?
Are all bent molecules polar? Mostly, yes. As aforesaid, bent molecules are asymmetrical just like trigonal pyramids and that means that they are polar molecules.
Which shape is always polar?
Lewis Structures and the Shapes of MoleculesFormula3D Structure Shape Polarity1.CH4tetrahedral nonpolar2.NH3trigonal pyramidal polar3.H2Obent polar4.H3O+trigonal pyramidal charged2 more rows
Is square pyramidal polar?
0:012:01Square Pyramidal Molecular Geometry/Shape and Bond AnglesYouTubeStart of suggested clipEnd of suggested clipBecause it has those five atoms.MoreBecause it has those five atoms.
What is the bond angle for octahedral structure?
VSEPR NotationNumber of Electron GroupsElectron-Group GeometryIdeal Bond Angles5trigonal-bipyramidal90°180°6octahedral90°90°9 more rows•Aug 21, 2020
How do I know if a bond is polar?
Although there are no hard and fast rules, the general rule is if the difference in electronegativities is less than about 0.4, the bond is considered nonpolar; if the difference is greater than 0.4, the bond is considered polar.
Which of the following is non-polar?
HF, HCl, HBr, HI.
What is the base bond angle of a molecular geometry?
If there are no lone pairs then the molecular geometry matches the electronic and is octahedral. The base bond angles are 180° and 90°. There is no reason to tweak the bonds to other values.
Do compounds exist in T-shaped?
Just like T-shaped in this category, no known compounds exist. In theory, if there were four lone pairs on this electronic geometry they would be in a planar arrangement which would leave the molecular geometry to be perfectly linear.
What is the octahedral shape?
The Octahedral shape is a type of shape which a molecule takes form of when there are 6 bonds attached to a central atom with 4 on the same plane. There are no lone pairs attached to it. The bond angle between the bonds is exactly 90 degrees. The molecule is non-polar since it is a symmetrical shape. Here are some examples of Octahedral-shaped molecules:
What is the shape of a molecule?
The square pyramidal shape is basically an Octahedral shape with 1 less bond. The angle between the bonds is 90 degrees and 84.8 degrees. The shape is polar since it is asymmterical. Here are some examples of Square Pyramidal-shaped molecules:
What is a square planar shape?
The Square planar shape is a type of shape which a molecule takes form of when there are 4 bonds attached to a central atom along with 2 lone pairs. If you were to remove 2 bonds from an Octahedral molecule and 1 bond from a Square Pyramidal molecule, it would form a square planar shape. The angle between the bonds is 90 degrees. The shape is non-polar since it is symmetrical. Here are some examples of Square planar-shaped molecules:
Overview
In chemistry, octahedral molecular geometry describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no b…
Isomerism in octahedral complexes
When two or more types of ligands (L , L , ...) are coordinated to an octahedral metal centre (M), the complex can exist as isomers. The naming system for these isomers depends upon the number and arrangement of different ligands.
For ML 4L 2, two isomers exist. These isomers of ML 4L 2 are cis, if the L ligands are mutually adjacent, and trans, if the L groups are situated 180° to each other. It was the analysis of such c…
Deviations from ideal symmetry
The term can also refer to octahedral influenced by the Jahn–Teller effect, which is a common phenomenon encountered in coordination chemistry. This reduces the symmetry of the molecule from Oh to D4h and is known as a tetragonal distortion.
Some molecules, such as XeF6 or IF 6, have a lone pair that distorts the symmetry of the molecule from Oh to C3v. The specific geometry is known as a monocapped octahedron, since it is derive…
Bioctahedral structures
Pairs of octahedra can be fused in a way that preserves the octahedral coordination geometry by replacing terminal ligands with bridging ligands. Two motifs for fusing octahedra are common: edge-sharing and face-sharing. Edge- and face-shared bioctahedra have the formulas [M2L8(μ-L)]2 and M2L6(μ-L)3, respectively. Polymeric versions of the same linking pattern give the stoichiometries [ML2(μ-L)2]∞ and [M(μ-L)3]∞, respectively.
Trigonal prismatic geometry
For compounds with the formula MX6, the chief alternative to octahedral geometry is a trigonal prismatic geometry, which has symmetry D3h. In this geometry, the six ligands are also equivalent. There are also distorted trigonal prisms, with C3v symmetry; a prominent example is W(CH3)6. The interconversion of Δ- and Λ-complexes, which is usually slow, is proposed to proceed via a trigonal prismatic intermediate, a process called the "Bailar twist". An alternative pathway for the
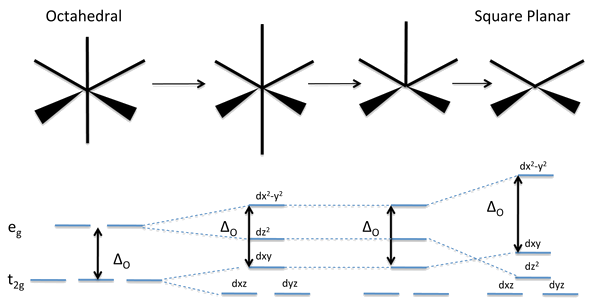
Splitting of the energy of the d-orbitals in octahedral complexes
For a free ion, e.g. gaseous Ni or Mo , the energy of the d-orbitals are equal in energy; that is, they are "degenerate". In an octahedral complex, this degeneracy is lifted. The energy of the dz and dx −y , the so-called eg set, which are aimed directly at the ligands are destabilized. On the other hand, the energy of the dxz, dxy, and dyz orbitals, the so-called t2g set, are stabilized. The labels t2g and eg refer to irreducible representations, which describe the symmetry properties of these …
Reactions
Given that a virtually uncountable variety of octahedral complexes exist, it is not surprising that a wide variety of reactions have been described. These reactions can be classified as follows:
• Ligand substitution reactions (via a variety of mechanisms)
• Ligand addition reactions, including among many, protonation
See also
• Octahedral clusters
• AXE method
• Molecular geometry