Is NaCl an ionic dipole force?
Ion-Dipole Forces are involved in solutions where an ionic compound is dissolved into a polar solvent, like that of a solution of table salt (NaCl) in water. Note, these must be for solutions (and not pure substances) as they involve two different species (an ion and a polar molecule). Click to see full answer. Thereof, is NaCl a dipole?
What is the ion-dipole interaction between sodium ion and chloride ion?
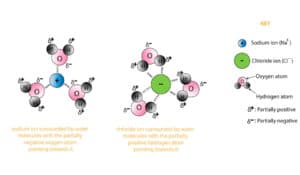
The image shows the ion-dipole interaction between sodium ion and chloride ion when immersed in water. For example, consider a sodium chloride molecule. When NaCl is dissolved in water, H20 has polar molecules, and this polar molecule is attracted towards Cl- and Na+ ions.
How are NaCl and NaCl ionic bonds formed?
The Na+ And Cl− Ions In NaCl Are Bonded Through An Electrostatic Force Of Attraction Commonly Known As The Ionic Bond. Water Is A Polar Solvent. The Oxygen Atom, Being More Electronegative, Attracts The Electron Cloud Toward Itself.
What type of intermolecular force is in NaCl?
The Na+ And Cl− Ions In NaCl Are Bonded Through An Electrostatic Force Of Attraction Commonly Known As The Ionic Bond. Water Is A Polar Solvent. The Oxygen Atom, Being More Electronegative, Attracts The Electron Cloud Toward Itself. One may also ask, what intermolecular force is in NaCl?
Does NaCl have ion-dipole interactions?
Does NaCl have ion ion forces?
What intermolecular forces is NaCl?
What is an example of ion-dipole forces?
Does NaCl have strong intermolecular forces?
Is NaCl a nonpolar molecule?
How does NaCl change intermolecular forces?
Is HCl a dipole?
HCl molecules, for example, have a dipole moment because the hydrogen atom has a slight positive charge and the chlorine atom has a slight negative charge.
What type of IMFA is nh3?
What are the 3 types of intramolecular forces?
What is the difference between ion and dipole?
The ion-dipole interaction is quite similar to dipole-dipole interaction. The only difference is that the bonds are formed between ions and polar molecules. The image shows the ion-dipole interaction between sodium ion and chloride ion when immersed in water.
Why is a nonpolar molecule an induced dipole?
The non-polar molecule becomes an induced dipole, due to the presence of an ion. This interaction between the ion and nonpolar molecules is called an ion-induced dipole interaction. The intermolecular forces strength depends upon the ease with which the non-polar molecule gets polarized.
What is the force between NaCl and H20?
When NaCl is dissolved in water, H20 has polar molecules, and this polar molecule is attracted towards Cl- and Na+ ions. The strength of the forces between them depends on the size of the polar molecule, and the strength of the dipole moment.
Which intermolecular force is the best example of a dipole-dipole interaction?
For Example, HCl shows the best intermolecular forces examples for a dipole-dipole interaction. In HCl, chlorine has a negative charge, and hydrogen has a positive charge. The attractive forces between the two opposite charges give rise to dipole-dipole forces. The magnitude of these forces can be predicted by the polarity of the molecules.
What is the force of attraction between atoms and molecules?
There always exists a force of attraction or repulsion between the atoms and molecules. The forces which exist in the molecule are responsible for the properties of the molecule. This can also be considered as an intermolecular forces definition.
What is the force that dissolves NaCl?
This attractive force is usually called an ion-dipole force. At the end, when all the NaCl dissolves, the sodium (Na +) and chloride (Cl –) ions will each be surrounded by water molecules and will appear at microscopic level as: Dissolved sodium chloride.
How does NaCl dissolve?
Sodium chloride (NaCl) dissolves when water molecules continuously attack the NaCl crystal, pulling away the individual sodium (Na +) and chloride (Cl –) ions. This nonstop attack continuous until the whole NaCl crystal disintegrates. To understand this process at the molecular level, we must apply the three steps we previously discussed.
What does the aqueous symbol mean in chemistry?
Therefore, when we write Na +(aq) or Cl –(aq) the symbol ( aq, aqueous) usually means that each ion is attracted to and surrounded by several water molecules.
Which atoms have a negative charge?
That’s one end (oxygen atom) of the water molecule carries a partial negative charge, while the other end (hydrogen atom) carries a partial positive charge. Because of this polarity, water molecules will arrange themselves such that the negatively charged oxygen atom will attract the positively charged sodium (Na +) ion, ...
Why do ions need to absorb energy?
These ions must absorb energy to increase their motion so that they can move away from each other. The water molecules must break the attraction between them so that they can make room to allow the sodium (Na +) and chloride (Cl –) ions to enter and interact with the water molecules.