Although iron is a good conductor of heat, it is not the best conductor and there are several alternatives to iron in the market as a better conductor such as copper, aluminum and brass.
Which is more thermal conductive copper or iron?
Copper wins the thermal conducity race by a factor of about 7 times that of iron. …in line with the relative electrical conductivity of both metals according to Weideman- Franz law [ 1] Is copper havan kund better or iron?
Why is copper not a good conductor of heat?
The grain structure of cu (copper) is such that when transmitting heat/electrons there is minor friction in between the grains. With out iron in copper, there is that much less resistance for heat/electrons to contend with. There are two kinds of brass. Yellow & red. Both are non-ferrous. They would be good conductors too.
Why is iron a good conductor of electricity?
Because the electrons involved in the metallic bond ofiron are free-moving, iron is a goodconductor. When in their liquid or gas states, the electrons ofIn order to conduct electricity, a substance needs to have chargesthat are free-moving.
Why are good electric conductors also good heat conductors?
Besides, the electrons has a contribution to the specific heat of materials by the same reason, so, in general good electric conductors are good heat conductors. Moreover, the atoms in a lattice also contribute to specific heat (even more than electrons dep
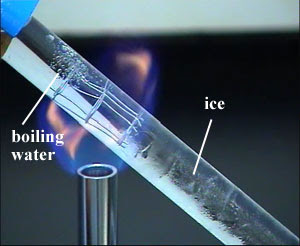
Is iron a good conductor of heat?
Heat is conducted through a material by means of vibration of the atoms. Good conductors such as iron have free electrons which can move around freely to transfer heat throughout the iron structure. So when iron is being heated, its free electrons vibrate quickly and conduct heat quicker.
Why is iron a good conductor of heat?
Iron is one of the commonly known metals that are used in many machineries and objects due to its good thermal conductive properties. Below are the reasons why iron is a good conductor of heat compared to other objects:
Is iron a better conductor of heat than copper, aluminum and brass?
Although iron is a good conductor of heat, it is not the best conductor and there are several alternatives to iron in the market as a better conductor such as copper, aluminum and brass.
Why does copper conduct heat better than iron?
According to the value of thermal conductivity, copper has the highest rating among all whereas iron falls way below copper in that regard. In the list below, you will get to know the reasons why copper conduct heat better than iron:
How does iron conduct heat?
Iron is being used in all sectors starting from machinery to household items due to its strength and thermal conductivity. Below are reasons explained as to why iron conducts heat so well:
Final Thoughts
Heat is carried through a medium by the vibration of its atoms. Iron, for example, has free electrons that may travel around freely to transport heat throughout the iron structure. As a result, when the iron is heated, its free electrons vibrate rapidly, making it a good conductor of heat.
Which metal is the best conductor?
Metals are good conductors as the electrons in their outer shell are loose and can plunge out of the atom with the application of the slightest force (voltage). Silver is the best metallic conductor, then comes copper. Why is one metal better at conduction than the other one?
What is the best electrical conduction?
The "best" electrical conduction is with superconductors - but of course then you need to consider the amount of current they can carry before they fail so "best" takes on a magnetic field dependence..."best" isn't a very scientific word is all I'm saying...there's almost always too many qualifications involved.
Why does iron have two different spin states?
First, because iron atoms indeed have individual magnetic moments, the overall band structure is split into two different spin states for the electrons. So, each spin state is separated from the others, reducing how each can move (relative to copper, were such splitting does not exist).
Is the Fermi surface a free electron sphere?
Second, and more importantly, the Fermi surface looks nothing like a free electron sphere. Instead, for iron the Fermi surface is not fully connected, comprising instead of multiple 'pockets' of electrons that do not communicate directly with the other pockets (and each spin state has a different set of pockets).
Is copper a noble metal?
Copper is a simple noble metal with a beautiful near-ideal Fermi surface (see Ashcroft and Mermin's Solid State Physics book, or C.Y. Fong et al., Comparison of band structures and charge distributions of copper and silver ).
Is silver the best conductor?
Stating categorically that silver is "the best" electrical conductor is more fan-boy enthusiasm than objective fact.
Is copper a Fermi surface?
It turns out that the Fermi surface of copper is almost exactly what one would expect from the free electron sphere. Electrons can move easily in any direction, the surface is fully connected, life is good. Iron is much more complicated.