HClO2 is a stronger acid than HClO. Click to see full answer. Furthermore, why is HClO2 stronger than HClO? The general rule is that the acid is stronger if it has more O atoms in a series such as this. HClO4, perchloric acid, is a very strong acid as is HClO3. HClO2 is a weak acid and HClO is even weaker. Thus we see more oxygen atoms means more possible strutures & which means stronger the acid.
Is HClO is the strongest acid?
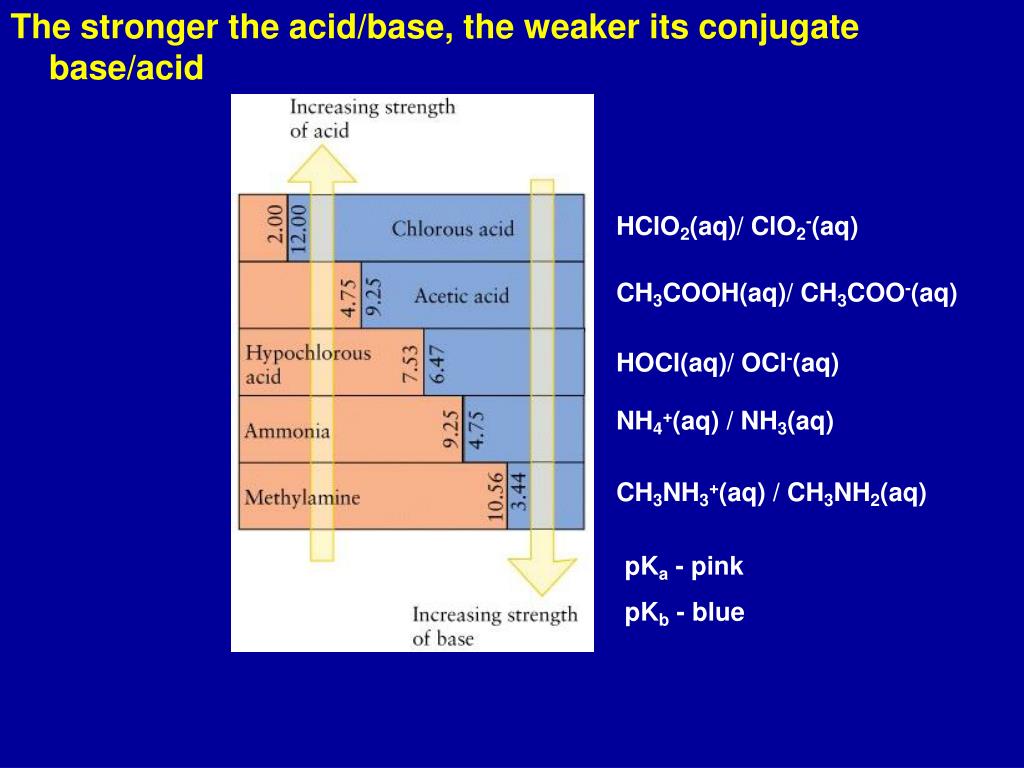
Thus, {ClO}- will be strongest base and so its conjugate acid HClO will be the weakest acid. Similarly, in this series {ClO4}- is the weakest base (maximum stabilized) and its conjugate acid HClO4 is the strongest acid. 110V 5L Home Oxygen Concentrator is on sale!
Is HClO2 a weak electrolyte?
– All other acids are weak acids/weak electrolytes: HF, HC2H3O2, HNO2, H2SO3, etc. Is BaCl2 an electrolyte? BaCl2 is a strong electrolyte, even stronger then NaCl (difference in electronegativity of 2.23). The substances which are completely ionized in dissolved or molten state, are known as strong electrolytes. Is na2so4 an electrolyte?
Which acid is stronger between HCl or H2SO4?
This is not a completely straightforward question. HCl and H2SO4 are categorized as strong acids. That means that they are about 100% ionized in water. In water all of the strong acids are of essentially the same strength. This happens because water is a leveling solvent. In water the strongest acid that can exist is H3O+, or hydronium ion.
Why is HCl a stronger acid than acetic acid?
HCL is stronger than acetic acid because it undergoes almost complete ionisation when dissolved in water(i.e. formation of H^+ and Cl^- ion) whereas when acetic acid is dissolved in water only 5% of it is dissociated into H^+ and CH3COOH^- ions.
Which one is the strongest acid HClO HClO2 HClO3 HClO4?
Thus, the order of acidic strength is HClO < HClO2 < HClO3 < HClO4 As the number of oxygen atoms attached to chlorine increases, acid strength increase.
Which is strongest HClO HClO2 HClO3?
The acid is much stronger if the number of oxygen atoms is more. In HClO3 and HClO2, HClO3 has more oxygen therefore it is more acidic.
Which among the two is stronger acid HClO2 or HOCl give reason?
HClO 2 is a little stronger than HClO because it contains two electronegative oxygens. Which is stronger oxidising agent HOCl or HClO4? Explanation: hocl is stronger oxidizing agent than hclo4 because it has the lowest oxidation state and hence it is a stronger oxidizing agent.Mar 1, 2020
Is HOCl more acidic than HClO2?
The decreasing order of acidic strength is HClO4>HClO3>HClO2>HClO.
Is HClO a weak acid?
HClO is a weak acid.
Which of the following is strongest acid HClO HClO2?
The HClO4 has the most oxygen atoms so it would have to be the strongest acid.
Is HClO a strong acid?
any acid that is not one of the seven strong is a weak acid (e.g. H3PO4, HNO2, H2SO3, HClO, HClO2, HF, H2S, HC2H3O2 etc.) 2. solutions of weak acids have a low concentration of H+.
Why HClO is stronger acid than HIO?
HCl is a weaker acid than HBr and HI due to the greater radii of Br and I, but why is HClO more acidic than HIO? Because Cl is more electronegative, it is more acidic.
Is HClO or HBrO a stronger acid?
In direct contrast with HCl vs. HBr , HClO is a stronger acid than HBrO , because Cl is more electronegative, which dominates over the size difference between Cl and Br due to the presence of the oxygen.Jan 10, 2018
Why is HClO weaker than HClO2?
The general rule is that the acid is stronger if it has more O atoms in a series such as this. HClO4, perchloric acid, is a very strong acid as is HClO3. HClO2 is a weak acid and HClO is even weaker. It can be simply explained by considering the stability of conjugate bases or corresponding anions.Mar 9, 2019
Which one is the strongest acid HClO?
The strongest acid is perchloric acid (HClO4 ).
Which is more acidic HOCl?
Originally Answered: Which is the strongest acid among HoCl, HoBr, and HoI? Strongest acid which liberates H+ easily or which doesn't liberate OH- easily . Since Cl is highly electronegative so HOCl is strongest acid.
What is the acidity of Cl in HClO4?
The acidity is decided on the basis of oxidation state on the central atom. Higher the oxidation state more the acidic nature. So, in HClO4 the oxidation state of Cl is +7. Similarly in HClO3 it's +5. In HClO2 it's +3. In HClO it's +1. So, amongst the given compounds most acidic is HClO4.
Why is the corresponding acid stronger?
Consequently, the corresponding acid will be strongest because weak conjugate base has strong acid and strong conjugate base has weak acid and vice versa.
What is the measure of acidity?
Acidic compounds dissociate in water or other solvents to produce free protons (H+). The concentration of these protons is what gives the acid its character. A measure of acidity or basicity is pKa, or acid dissociation constant. pKa= -log [H+], and a lower pKa means a higher concentration of protons and a stronger acid. HCl and H2SO4 are both strong acids, meaning that they dissociate completely in water as follows:
How many protons does H2SO4 have?
H2SO4 has 2 protons to lose. After the first one is separated, we have the HSO4- ion. It’s hard, energetically, to pull a positive proton off of a negative ion, so HSO4- is unlikely to progress to SO4 2-. So even though sulfuric acid has 2 protons to lose, really only about 1 is removed. HSO4- is actually a weak conjugate base, so it raises the pKa of H2SO4.
What happens to the acidic character of a halogen atom with an increase in oxidation number?
With increase in oxidation number of a particular halogen atom, the acidic character of corresponding oxoacid increases.
What order does acidic strength increase?
The acidic strength increases with the increase of +ve charge on cl atom. This increased positive charge makes The molecule overall more electronegative to drag the electrons from H- the smallest atom. So the order for acidic strength is -. Acidic strngth order - HClO4>HClO3>HClO2>HClO.
Which acid is the weakest base?
Thus, {ClO}- will be strongest base and so its conjugate acid HClO will be the weakest acid. Similarly, in this series {ClO4}- is the weakest base (maximum stabilized) and its conjugate acid HClO4 is the strongest acid.
Relative acidity of HClO vs HClO2 etc
Can someone provide more of the "why" in this explanation pasted below for the relative acidity of HClo, HClo2, etc.? My comments/questions are in italics
Re: Relative acidity of HClO vs HClO2 etc
In general, adding more oxygen atoms to the central atom in an oxyacid helps to distribute the negative charge of the conjugate base over a greater number of atoms. If a proton is less strongly attached to any one of the oxygens, then you get a stronger acid.
Which acid is the strongest?
As another answer pointed out, HClO4 is the strongest acid in the series because the negative charge on the conjugate base (ClO 4 −) is resonance-stabilized over all four oxygens.
Which is the strongest oxidant?
Based on these standard reduction potentials, in acidic medium, HClO2 is the strongest oxidant, followed by HClO (i.e., “bleach”).
What is acidity measured as?
Acidity can be measured as the easy of releasing a proton (H+) unit.
What happens when the central chlorine atom is attached to the electron withdrawing oxygen atom/s?
The more the electron withdrawing oxygen atoms, more will be the electron withdrawing effect. Once the electron on the bonded hydrogen atom is pulled towards the oxygen atom, the hydrogen atom can be removed as hydrogen ion. If more oxygen atoms are present, then the combined withdrawal effect makes it easier for the formation of hydrogen ion. Hence electron withdrawal increases strength of the acid.
Is ClO3 a resonance hybrid?
There is another factor stabilizing ClO3 (-) over ClO2 (-). These are both resonance hybrids (look here if you don’t understand this term). Resonance makes the three oxygen atoms in ClO3 (-) equal, so that each oxygen atom has -1/3 charge. Similarly, resonance gives each oxygen atom in ClO2 (-) a -1/2 charge. Since the negative charge is more spread out in ClO3 (-) than in ClO2 (-), this also makes ClO3 (-) a weaker conjugate base, and thus HClO3 the stronger acid,
Which is more electronegative, bromine or chlorine?
Chlorine being more electronegative than Bromine it pulls the electron of hydrogen more strongly and hence has a higher tendency to release a H+ unit. Hence we can s
Is hydrogen chloride the same as hydronium?
It depends on what your question really is. If you mean aqueous solutions, they are the same, as the acid is essentially hydronium, which is probably usually something like H5O2+. If you mean pure, hydrogen chloride in the liquid state is more difficult to consider because out is difficult to measure the acidity. Very strong acids need some other species to get acidity into some order, and the strength of very strong acids has traditionally been measured by their ability to stabilise carbenium ions, i.e. will it protonate the associated double bonded material? The problem with hydrogen chlorid
Why is the O-H bond stronger on HClO?
Hence, the O-H bond on HClO will be stronger and, therefore, that H will be more reluctant to be released as a proton (H⁺).
How many oxygen atoms are in HClo3?
Comparing HClo3 and HBrO3 there are 3 oxygen atoms bound to the central halogen in each case causing a shift of electrons from the halogens to the oxygen.
What happens when the central chlorine atom is attached to the electron withdrawing oxygen atom/s?
The more the electron withdrawing oxygen atoms, more will be the electron withdrawing effect. Once the electron on the bonded hydrogen atom is pulled towards the oxygen atom, the hydrogen atom can be removed as hydrogen ion. If more oxygen atoms are present, then the combined withdrawal effect makes it easier for the formation of hydrogen ion. Hence electron withdrawal increases strength of the acid.
Why do you attach more oxygen atoms to the central atom in an oxyacid?
Attaching more oxygen atoms to the central atom in an oxyacid helps to distribute the negative charge of the conjugate base over a greater number of atoms. This makes the proton less strongly attracted to any one of the oxygen atoms in the conjugate base. Hence, you get a stronger acid.
Why do oxyacids attach to more oxygen atoms?
Attaching more oxygen atoms to the central atom in an oxyacid helps to distribute the negative charge of the
What happens when an atom is attached to an OH bond?
Here, Electronegativity of atom X (F, Cl or Br or I) attached to O decreases down a group, polarity of OH bond decreases, acid strength decreases. It means more the electronegativity of the halogen atom, more the tendency to pull electron and weaker the O-H bond and hence proton (H+) can easily remove.
Which is more electronegative, bromine or chlorine?
Chlorine being more electronegative than Bromine it pulls the electron of hydrogen more strongly and hence has a higher tendency to release a H+ unit. Hence we can s