Those atoms can be the same element, as when oxygen bonds with itself to form O2, or with different elements, such as water (H2O). The exceptions to the octet rule are hydrogen and helium, which are both happy with two electrons in their outer shells.
Full Answer
Does HCl obey the octet rule?
Therefore HCl obeys the octet rule. (Refer to the structure in the attached image). The atomic number of chlorine is 17 and therefore it has seven valence electrons in it. The atomic number of carbon is 6 so it has four valence electrons in its valence shell. Each chlorine atom requires an electron to complete its octet.
Why does oxygen obey the octet rule but hydrogen does not?
The atomic number of hydrogen is 1 so it has only one valence electron in its valence shell. Oxygen needs two more electrons to fulfill its octet so it forms two single bonds with two hydrogen atoms. Therefore obeys the octet rule. (Refer to the structure in the attached image).
Which of the following compound is an exception to the octet rule?
The compound that is an exception to the octet rule is ClF3. Normally, chlorine atom needs only 1 electron to be filled. In here, it accepts three atoms of flourine to be completely stable.
What is the octet rule for sulfur?
Sulfur can follow the octet rule as in the molecule SF 2. Each atom is surrounded by eight electrons. It is possible to excite the sulfur atom sufficiently to push valence atoms into the d orbital to allow molecules such as SF 4 and SF 6. The sulfur atom in SF 4 has 10 valence electrons and 12 valence electrons in SF 6.
Does H2O obey octet rule?
Lewis Structure of H2O Look for the total valence electrons: It is eight to form a single H2O molecule. Look for how many electrons are needed: It is four for one water (H2O) molecule according to the octet rule. Find the total number of bonds forming: Single covalent bonds between each oxygen and hydrogen atom.
What are the 4 exceptions to the octet rule?
However, there are three general exceptions to the octet rule: Molecules, such as NO, with an odd number of electrons; Molecules in which one or more atoms possess more than eight electrons, such as SF6; and. Molecules such as BCl3, in which one or more atoms possess less than eight electrons.
What 3 elements are exceptions to the octet rule?
Hydrogen, beryllium, and boron have too few electrons to form an octet. Hydrogen has only one valence electron and only one place to form a bond with another atom. Beryllium has only two valence atoms, and can form only electron pair bonds in two locations. Boron has three valence electrons.
What 2 elements are exceptions to the octet rule?
An octet is the eight-electron arrangement in the outer electron shell of the noble-gas atoms. The core beryllium and boron atoms in the two molecules represented in this image have less than eight valence electrons and hence do not meet the octet rule.
Which molecules do not follow the octet rule?
While most atoms obey the duet and octet rules, there are some exceptions. For example, elements such as boron or beryllium often form compounds in which the central atom is surrounded by fewer than eight electrons (e.g., BF₃ or BeH₂).
Does oxygen follow the octet rule?
The molecules of the halogens, oxygen, nitrogen, and carbon are known to obey the octet rule. In general, the elements that obey this rule include the s-block elements and the p-block elements (except hydrogen, helium, and lithium).
What is the Lewis dot structure for h2o?
0:001:21How to Draw the Lewis Structure for Water - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo 1 times 2 is 2 plus 6 2 plus 6 equals 8. We have a total of 8 valence electrons. We'll put theMoreSo 1 times 2 is 2 plus 6 2 plus 6 equals 8. We have a total of 8 valence electrons. We'll put the oxygen in the center and hydrogens always go on the outside. And then we'll put a pair of electrons.
Does co2 follow the octet rule?
In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in both the carbon octet and the oxygen octet, so that both atoms are considered to obey the octet rule.
What is the octet rule?
Todd Helmenstine. Updated August 04, 2019. The octet rule is a bonding theory used to predict the molecular structure of covalently bonded molecules. According to the rule, atoms seek to have eight electrons in their outer—or valence—electron shells.
Which molecule follows the octet rule?
Sulfur and phosphorus are common examples of this behavior. Sulfur can follow the octet rule as in the molecule SF 2. Each atom is surrounded by eight electrons. It is possible to excite the sulfur atom sufficiently to push valence atoms into the d orbital to allow molecules such as SF 4 and SF 6. The sulfur atom in SF 4 has 10 valence electrons ...
What is a compound with an odd number of electrons called?
There is a class of compounds where the valence electrons contain an odd number of electrons in the valence shell. These molecules are known as free radicals. Free radicals contain at least one unpaired electron in their valence shell. In general, molecules with an odd number of electrons tend to be free radicals.
Which rule has more elements breaking the rule than following it?
And the octet rule has more elements breaking the rule than following it. While Lewis electron dot structures help determine bonding in most compounds, there are three general exceptions: molecules in which atoms have fewer than eight electrons (boron chloride and lighter s- and p- block elements); molecules in which atoms have more ...
Which molecules have an odd number of electrons?
In general, molecules with an odd number of electrons tend to be free radicals. Nitrogen (IV) oxide (NO 2) is a well-known example. Note the lone electron on the nitrogen atom in the Lewis structure. Oxygen is another interesting example. Molecular oxygen molecules can have two single unpaired electrons.
Which element has too few electrons?
Too Few Electrons: Electron Deficient Molecules. Hydrogen, beryllium, and boron have too few electrons to form an octet. Hydrogen has only one valence electron and only one place to form a bond with another atom. Beryllium has only two valence atoms, and can form only electron pair bonds in two locations.
What is the orbital of an element in a period greater than period 3?
Elements in periods greater than period 3 on the periodic table have a d orbit al available with the same energy quantum number. Atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons.
Example
The formula for table salt is NaCl. It is the result of Na + ions and Cl - ions bonding together. If sodium metal and chlorine gas mix under the right conditions, they will form salt. The sodium loses an electron, and the chlorine gains that electron. In the process, a great amount of light and heat is released.
Two Electrons
The main exception to the rule is hydrogen, which is at its lowest energy when it has two electrons in its valence shell. Helium (He) is similar in that it, too, only has room for two electrons in its only valence shell.
Less Than an Octet
Other notable exceptions are aluminum and boron, which can function well with six valence electrons. Consider BF 3</sup>. The boron shares its three electrons with three fluorine atoms. The fluorine atoms follow the octet rule, but boron has only six electrons.
More Than an Octet
In Period 3, the elements on the right side of the periodic table have empty d orbitals. The d orbitals may accept electrons, allowing elements like sulfur, chlorine, silicon and phosphorus to have more than an octet. Compounds such as PCl 5 and SF 6 can form. These compounds have 10 and 12 electrons around their central atoms, respectively.
Odd Numbers
Some elements, notably nitrogen, have an odd number of electrons and will form somewhat stable compounds. Nitric oxide has the formula NO. No matter how electrons are shared between the nitrogen and oxygen atoms, there is no way for nitrogen to have an octet. It will have seven electrons instead.
What are the exceptions to the octet rule?
Some of the exceptions about octet rule are given below: An electron or molecule which contains unpaired electrons in its outermost shell or valence shell is considered as free radical. These electrons are less stable and do not obey the octet rule.
What is the octet rule?
The octet rule states that the elements which can lose, gain, or share electrons from its outermost shell to complete the valence shell with a set of eight electrons. Valence electrons mean the total number of electrons present in the outermost shell of an element that can participate in the bond formation. ...
What is the difference between an octet and a valence electron?
What is the Difference Between the Octet of an Electron and a Valence Electron? An octet of an electron means the presence of eight electrons in its outermost shell. This is the ability of an electron to gain, lose, or share their electrons with other elements to complete its octet. The valence of an electron means the total number ...
How many electrons does carbon have?
Carbon contains four electrons in its outermost shell. Also, carbon should have four electrons to complete its octet when it is combining with two molecules of oxygen. Here each carbon atom requires two electrons to complete its octet. Carbon and oxygen share their outermost electron and form CO2 which further completes the octet.
How many electrons can a d-orbital hold?
Another exception of octet rule is transition elements. Due to the presence of d-orbitals, they can hold 18 electrons in its outermost shell.
Which element has seven electrons in its outermost shell?
Carbon and oxygen share their outermost electron and form CO2 which further completes the octet. 2. NaCl. Chlorine contains seven electrons in its outermost shell and requires only one electron to complete its octet whereas sodium contains one electron in its outermost shell.
Which elements obey the octet rule?
Elements that obey octet rules are main group elements which are oxygen, carbon, nitrogen. s-block and p-block elements obey octet rule except for hydrogen, helium, and lithium.
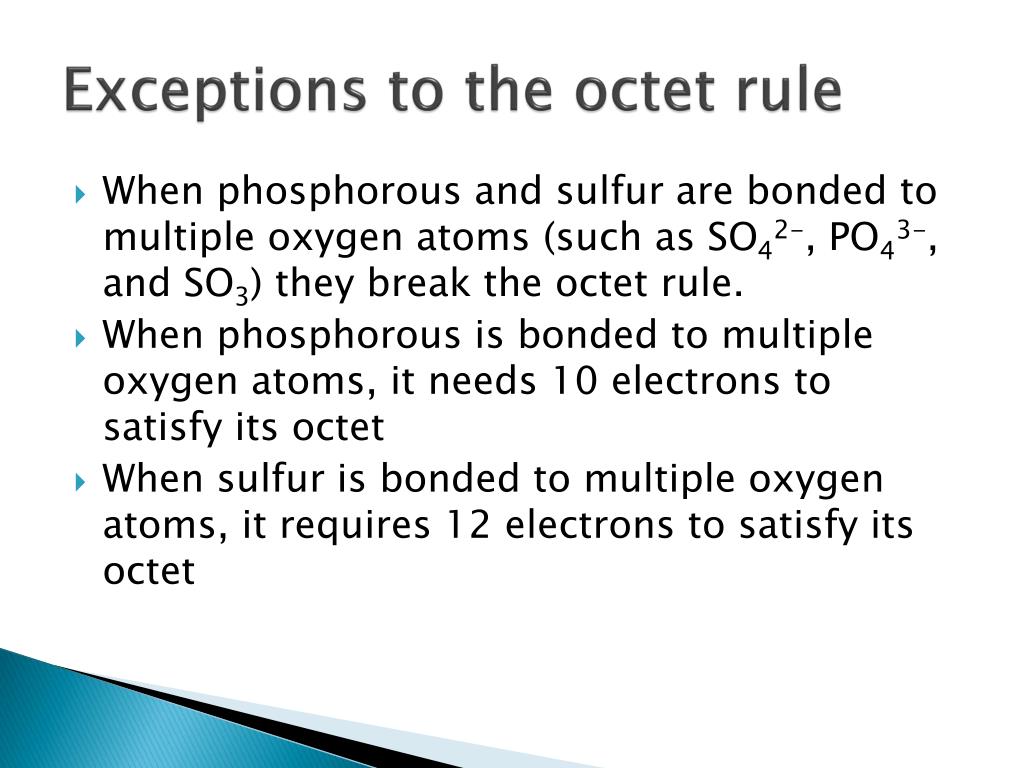
How many electrons are in the Lewis structure of PO43?
The total number of electrons to be counted for the Lewis structure of the PO43- polyatomic ion is: 32. The central atom in the chlorate anion, ClO3- is surrounded by: three bonding and one unshared pair of electrons.
Which is more electronegative, chlorine or hydrogen?
Hydrogen is more electronegative and the shared electron pair is likely to be found on the hydrogen atom. Chlorine is more electronegative and the shared electron pair is likely to be found on the hydrogen atom. Chlorine is more electronegative and the shared electron pair is likely to be found on the chlorine atom.
Is water a polar molecule?
A water molecule is asymmetric and therefore is polar. A water molecule is symmetrical and therefore is nonpolar. A water molecule has two dipole moments and they cancel each other. The electronegativities of hydrogen and oxygen are equal and therefore a water molecule is nonpolar.
Is a duet ionic or covalent?
All of the above statements are true. A duet is a stable electron configuration for helium. An ionic bond occurs when electrons are transferred. An octet is when an atom has 8 valence electrons. A covalent bond occurs when electrons are shared. All of the above statements are true.