Full Answer
Is CH4 a molecule or formula unit?
NaCl is its formula unit. That formula unit consists of sodium cation and chloride. Methane is a covalent compound. Its formula unit is CH4 (4 as a subscript). That formula unit is a molecule of CH4. When Ionic compounds dissolve in water, the dissociate into ions.
How many molecules of CH4 are in a mole of CH4?
Best Answer. A mole is Avogadro's number worth, so there are approximately 3*6.022×10 23 molecules of CH4 and 5 times that number of atoms in 3 moles of CH4.
What is the structural formula for CH4?
- Identify central atom & valence electrons.
- Add one for each single bond & none for double bonds.
- Add one for every unit negative charge & subtract one for every unit positive charge.
What is the molecular shape of CH4?
To summarize this blog, we can conclude the following points for the Methane molecule:
- CH4 molecule is made up of one Carbon atom and four hydrogen atoms.
- There are four single bonds in the molecule between Carbon and Hydrogen atoms.
- A total of 8 valence electrons are there for this molecule.
- All the valence electrons are used up in the bond formation; hence the molecule has no lone pairs, and there are four bonding pairs of electrons.
See more
What is the unit of CH4?
METHANE FLOW RATES (METERED SYSTEMS) Standard cubic meters per minute: Cubic meters per minute at 15°C and 100 kilo-pascal pressure. Normal cubic meters per minute: Cubic meters per minute at 0°C and 101.325 kilo-pascal pressure.
Is molecule a unit?
molecule, a group of two or more atoms that form the smallest identifiable unit into which a pure substance can be divided and still retain the composition and chemical properties of that substance.
What is the formula of CH4?
CH₄Methane / Formula
Is h2o a molecule?
Water as a Compound and Molecule The chemical formula for water is H2O, which means each molecule of water consists of one oxygen atom chemically bonded to two hydrogen atoms. Thus, water is a compound. It's also a molecule, which is any chemical species formed by two or more atoms chemically bonded to each other.
Which of the following is not a molecule?
What Is Not a Molecule? Single atoms of elements are not molecules. A single oxygen, O, is not a molecule.
Is methane a molecule?
Methane is a molecule composed of one carbon atom linked to four hydrogen atoms.
Is methane a molecular?
Methane (US: /ˈmɛθeɪn/ MEH-thayn, UK: /ˈmiːθeɪn/ MEE-thayn) is a chemical compound with the chemical formula CH4 (one carbon atom bonded to four hydrogen atoms)....Methane.NamesChemical formulaCH4Molar mass16.043 g·mol−1AppearanceColorless gasOdorOdorless62 more rows
How many atoms are in a molecule of CH4?
One methane molecule, CH4, contains one carbon atom and four hydrogen atoms.
What is CH4 in chemical terms?
CH4 is the chemical formula for Methane. Four Hydrogen atoms covalently bonded to one Carbon atom. Covalently bonded substances exist as individual particles called molecules.
How many hydrogen atoms are in CH4?
CH4 states that there are 4 hydrogen atoms (H4) covalently bonded (covalent bond meaning an atom shares valence electrons with another atom in order to become more stable) to a single carbon atom (C) and therefore acts as one entity until these bonds are broken. The definition of a molecule being a group of atoms bonded together, makes CH4 a molecule.
What is a group of atoms bonded together?
a molecule is, a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction.
What is CH4 gas?
It is a molecule called methane or natural gas.CH4 is methane, a colorless, odorless gas. It is the simplest of hydrocarbons.
What type of bond is found in methane?
The chemical bonds found within methane are classified as covalent bonds. A covalent bond is formed when two or more atoms share one or more electrons located in the outermost energy level, also known as the valenceshell. The valence electrons from each donor atom form pairs through overlap of their electron clouds to create a covalent bond.
How do covalent bonds form in methane?
For methane the covalent bonds form from the sharing of a single electron from each hydrogen with the four unpaired valence electrons of a single carbon atom. The hydrogen atoms are arranged around the central carbon atom in a geometry known as a tetrahedral geometry. Tetrahedral geometry means that, if you drew a line to connect three hydrogen atoms on the same side of the molecule, you would have a pyramid with four triangular faces.
Is CH4 a gas?
CH4 is methane, a colorless, odorless gas. It is the simplest of hydrocarbons, as it contain only 1 carbon atom. Furthermore, it’s electronic and geometric configurations are both tetrahedral. It’s very stable, containing 4 carbon-hydrogen polar covalent bonds. However, it is without a permanent dipole moment due to its geometry - the individual bond moments sum vectorially to zero.
What are the properties of CH4?
Properties of Methane – CH4. Methane is one of the most important greenhouse gasses and approximately 70% of methane emissions are linked to human activities. Pure methane is an energy-rich feed stock with an energy density of 55.7 MJ/kg and is used to generate electricity, for domestic heating and cooking. CH 4.
What is CH4 used for?
CH4 Uses (Methane) It is used in automobiles, ovens and water heater as a fuel. It is used in the generation of electricity. It is used as rocket fuel in its refined liquid form. It is used as an antifreeze ingredient in industries. It is a common ingredient in fertilizer. It is used to sanitize products.
What is the simplest form of methane?
What is Methane? Methane is a simplest of the saturated hydrocarbons with a chemical formula CH 4. It consists of four hydrogen atoms and one carbon atom and is the simplest alkane. When natural methane reaches the surface of the atmosphere is called atmospheric methane and can be found under the seafloor as well as below the ground.
Is methane a flammable gas?
It is a flammable non-toxic gas. It is a tetrahedral molecule which has four equivalent C-H bonds. It is produced by colonic anaerobes. Alessandro Volta an Italian physicist was the first to scientifically identify methane in the year 1776.
Is methane a volatile or nonvolatile gas?
Fossil fuels range from volatile materials with low carbon-to-hydrogen ratios (like methane), to liquids (like petroleum), to almost pure carbon-composed non-volatile materials, such as anthracite coal. Methane can be found either alone, in combination with oil, or in the form of methane clathrates in hydrocarbon fields.
What is the chemical formula for methane?
Methane ( US: / ˈmɛθeɪn /, UK: / ˈmiːθeɪn /) is a chemical compound with the chemical formula CH4 (one atom of carbon and four atoms of hydrogen ). It is a group-14 hydride and the simplest alkane and is the main constituent of natural gas.
How many molecular orbitals does methane have?
Methane is a tetrahedral molecule with four equivalent C–H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H. The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on ...
How much energy is in a cubic meter of natural gas?
In this context it is usually known as natural gas, which is considered to have an energy content of 39 megajoules per cubic meter, or 1,000 BTU per standard cubic foot. Liquefied natural gas (LNG) is predominantly methane (CH 4) converted into liquid form for ease of storage or transport.
What is the boiling point of methane?
Methane has a boiling point of −161.5 °C at a pressure of one atmosphere.
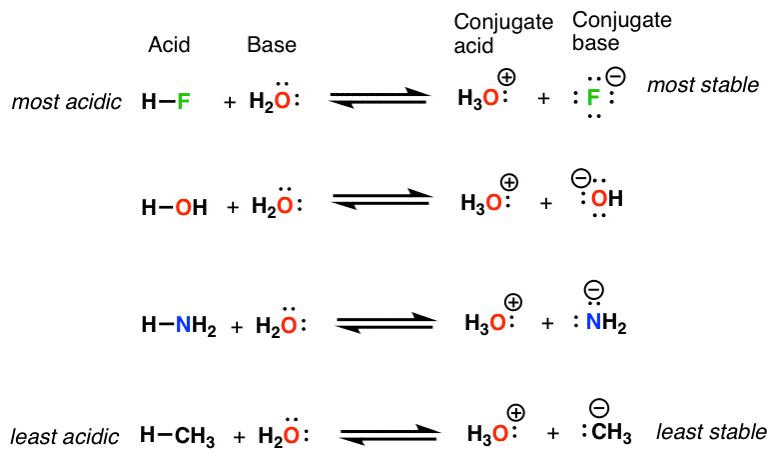
Is methane a weak acid?
Like other hydrocarbons, methane is a very weak acid. Its pK a in DMSO is estimated to be 56. It cannot be deprotonated in solution, but the conjugate base is known in forms such as methyllithium .
Who discovered methane?
In November 1776, methane was first scientifically identified by Italian physicist Alessandro Volta in the marshes of Lake Maggiore straddling Italy and Switzerland. Volta was inspired to search for the substance after reading a paper written by Benjamin Franklin about "flammable air". Volta collected the gas rising from the marsh, and by 1778 had isolated the pure gas. He also demonstrated that the gas could be ignited with an electric spark.
Is methane a liquid?
Uses. Methane is used in industrial chemical processes and may be transported as a refrigerated liquid (liquefied natural gas, or LNG ). While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas, the gas at ambient temperature is lighter than air.
What is the rate constant for the vapor phase reaction of methane with photochemically produced hydroxyl radical?
The rate constant for the vapor-phase reaction of methane with photochemically-produced hydroxyl radicals is 6.86X10-15 cu cm/molecule-sec at 25 °C (1). This corresponds to an atmospheric half-life of about 4 years at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm (2). Methane is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions (3). Methane does not contain chromophores that absorb at wavelengths >290 nm (3) and, therefore, is not expected to be susceptible to direct photolysis by sunlight (SRC).
What is activated charcoal used for?
Activated charcoal is used as a gastric decontamination agent in emergency clinical setting s in case of poison or medication overdose. Studies show that early administration of one dose of activated charcoal can adsorb poison in the stomach and reduce absorption while it also works long after ingestion, by interruption of enterohepatic and enterovascular cycling of poison.
Is a mononuclear parent hydride a compound?
It is a mononuclear parent hydride, a one- carbon compound, a gas molecular entity and an alkane. It is a conjugate acid of a methanide. Natural gas, refrigerated liquid (cryogenic liquid) appears as a flammable liquefied gaseous mixture of straight chain hydrocarbons, predominately methane. CAMEO Chemicals.
Is methane a gas?
Methane is the principal constituent of natural gas (1,3); natural gas from America is approximately 85% methane (4). Emissions of geothermal steam may release methane to the environment (1,5). Methane is a constituent of petroleum gases (6).
What is the formula weight of a compound?
The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction.
How to calculate molar mass?
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
How to find the formula weight?
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Is molar mass the same as molecular mass?
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight ...
Overview
Methane is a chemical compound with the chemical formula CH4 (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under norma…
Properties and bonding
Methane is a tetrahedral molecule with four equivalent C–H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H. The lowest-energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms. Above this energy level is a triply degenerate set of MOs that involve overlap of the 2p orbitals on carbon with vario…
Chemical reactions
The primary chemical reactions of methane are combustion, steam reforming to syngas, and halogenation. In general, methane reactions are difficult to control.
Partial oxidation of methane to methanol, a more convenient, liquid fuel, is challenging because the reaction typically progresses all the way to carbon dioxide and water even with an insufficient supply of oxygen. The enzyme metha…
Uses
Methane is used in industrial chemical processes and may be transported as a refrigerated liquid (liquefied natural gas, or LNG). While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas, the gas at ambient temperature is lighter than air. Gas pipelines distribute large amounts of natural gas, of which methane is the principal component.
Generation
The two main routes for geological methane generation are (i) organic (thermally generated, or thermogenic) and (ii) inorganic (abiotic). Thermogenic methane occurs due to the breakup of organic matter at elevated temperatures and pressures in deep sedimentary strata. Most methane in sedimentary basins is thermogenic; therefore, thermogenic methane is the most important source …
Occurrence
Methane was discovered and isolated by Alessandro Volta between 1776 and 1778 when studying marsh gas from Lake Maggiore. It is the major component of natural gas, about 87% by volume. The major source of methane is extraction from geological deposits known as natural gas fields, with coal seam gas extraction becoming a major source (see coal bed methane extraction, a meth…
History
In November 1776, methane was first scientifically identified by Italian physicist Alessandro Volta in the marshes of Lake Maggiore straddling Italy and Switzerland. Volta was inspired to search for the substance after reading a paper written by Benjamin Franklin about "flammable air". Volta collected the gas rising from the marsh, and by 1778 had isolated pure methane. He also demonstrated that th…
Etymology
Etymologically, the word "methane" is coined from the chemical suffix "-ane", which denotes substances belonging to the alkane family; and the word "methyl", which is derived from the German "methyl" (1840) or directly from the French "méthyle", which is a back-formation from the French "méthylène" (corresponding to English "methylene"), the root of which was coined by Jean-Baptiste Dumas and Eugène Péligot in 1834 from the Greek "methy" (wine) (related to English "m…