Is CaCl2 ionic or covalent bond?
22/03/2020 · Is calcium chloride an ionic or covalent bond? Yes,calcium chloride is ionic. CaCl2, is a salt of calcium and chlorine. It behaves as a typical ionic halide and is solid at room temperature. Calcium is a metal which is bonded to non-metal sulfate. Click to see full answer.
What is the ionic bond between calcium and chlorine?
Answer: Calcium chloride ( CaCl2 ) is ionic bond What is chemical bond, ionic bond, covalent bond? Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing …
What is the electronegativity difference of calcium chloride?
2 days ago · In summary, calcium chloride is an ionic compound owing to the large electronegativity difference of the Ca-Cl bond in calcium chloride, which is greater than 2.0. The calcium atom forms a positive calcium ion by losing two electrons and the chlorine atom forms a negative chlorine ion by accepting one electron.
How do you know if a compound is ionic or covalent?
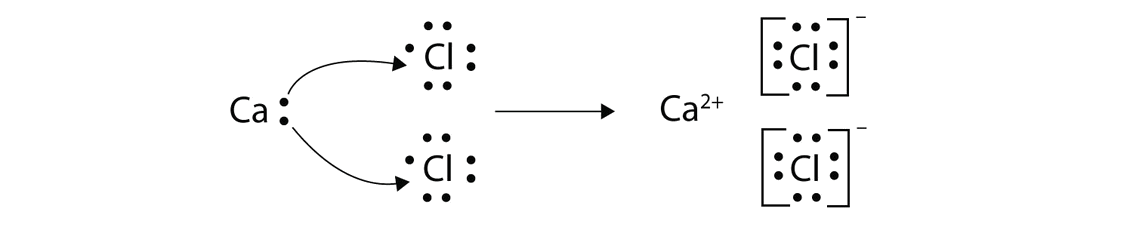
23/11/2016 · Another example of electrovalent or ionic bonding is calcium chloride. Before combination (bonding), calcium has 20 proton (atomic mass) I.e 20 electrons with electronic configuration of 2,8,8,2. And chlorine has 17 proton (atomic mass) I.e 17 electrons with electronic configuration of 2,8,7.
What type of bond is calcium chloride?
ionic bondsCalcium chloride is created from the ionic bonds that form between calcium cations and chloride anions. Calcium ions have a charge of +2, while chloride ions have a charge of -1.16-Sept-2021
Why is calcium chloride an ionic bond?
Ionic compounds generally form between elements that are metals and elements that are nonmetals. For example, the metal calcium (Ca) and the nonmetal chlorine (Cl) form the ionic compound calcium chloride (CaCl2). ... Because the positive and negative charges cancel out, an ionic compound is neutral in charge.
Does calcium chloride contain a covalent bond?
0:151:23Is CaCl2 (Calcium chloride) Ionic or Covalent? - YouTubeYouTubeStart of suggested clipEnd of suggested clipThat is going to be an ionic. Compound.MoreThat is going to be an ionic. Compound.
Is calcium chloride a cation or an anion?
Calcium chloride is an important calcium salt that has many household and industrial applications. Formula and structure: The chemical formula of calcium chloride is CaCl2, and its molar mass is 110.983 g/mol. It is an ionic compound consisting of the calcium cation (Ca2+) and two chlorine anions (Cl-).
Why do nonmetals have ionic bonds?
Hence, ionic bond results when there is transferring of electron (s) either from one atom to another atom or from metal to nonmetal.
What is the electronegativity of a chlorine atom?
The electronegativity value of the chlorine atom = 3.16. The electronegativity difference of the Ca-Cl bond = 2.16. The electronegativity difference of the Ca-Cl bond in the calcium chloride compound is 2.16 on the Pauling scale, which is greater than 2.0 and confirms the ionic nature of the Ca-Cl bond.
How are chemical bonds formed?
A chemical bond is formed either by transferring electrons or by sharing electrons between atoms of the molecule. The chemical bond is formed by the participation of only valence electrons, electrons of the outermost shell, of the atom. There are two types of chemical bonds based on their formation. Covalent Bond. Ionic Bond.
Is a covalent bond polar or nonpolar?
However, this sharing may be equal or unequal depending on the electronegativity of the constituent atoms of the molecules. Hence, a covalent bond may be polar or nonpolar. In covalent bonding, the ionization energy ...
Is a chemical bond ionic or covalent?
A chemical bond is an Ionic bond if the electronegativity difference of the chemical bond is greater than 2.0 and it will be a covalent bond if the electronegativity difference is less than 2.0 on the Pauling scale.
Is calcium chloride soluble in water?
Calcium chloride is highly soluble in water owing to its ionic nature. Anhydrous calcium chloride crystallizes in the orthorhombic and tetragonal structure whereas hexahydrate calcium chloride crystallizes itself in trigonal structure.
Is calcium chloride an ionic compound?
In summary, calcium chloride is an ionic compound owing to the large electronegativity difference of the Ca-Cl bond in calcium chloride, which is greater than 2.0. The calcium atom forms a positive calcium ion by losing two electrons and the chlorine atom forms a negative chlorine ion by accepting one electron.
What is the bonding of atoms?
Atoms of an element combine with one another in various ways and this combination are called bonding. Chemical bonding of ionic and covalent bonds. There are many different types of bonding of which covalent bonding, electrovalent or ionic bonding, co-ordinate covalency or co-ionic bonding, hydrogen bonding, metallic bonding ...
How many electrons does calcium need to bond with chlorine?
That means, Calcium is looking for 6 electrons and chlorine is looking for one electron to obey octet rule. In order for bonding to take place, the two valency of calcium will be transferred to chlorine atoms so that chlorine can also obey octet rule. When this happens, the two elements becomes bonded together.
Is aluminum a metal or a nonmetal?
Yes aluminum is a metal and chlorine is a non-metal, but have you also forget that aluminum is an element of semiconductor, that is under physics, let just agree in chemistry that aluminium is a semi-metal. You will know more on types of elements and groups when get to periodic table.#N#But please note what is above, the bond between Aluminium and Chlorine to form Aluminium Chloride (AlCl3) is covalent bond not ionic bond
What is the most important principle of ionic bonding?
Ionic or electrovalent bonding is the type of chemical bonding between metal and non-metal and the most important principle in ionic bond is that, it’s a donor-acceptor principle in which there is a complete electron transfer and the atoms formed are usually present as ions. During ionic bonding, the donor atom is usually a metal ...
What happens to the donor atom during ionic bonding?
During ionic bonding, the donor atom is usually a metal of relatively big size and the acceptor atom is usually a non-metal and relatively small size. During the bonding, an atom of a metallic element or group loses electrons from its outermost shells. The number of electrons is equal to number of its valency to attain a stable configuration ...
What are some examples of ionic bonds?
When this happens, the two elements becomes bonded together. Other examples of ionic bonding is calcium oxide (CaO), Potassium chloride (KCl), just have it in mind that ionic bonding occurs between metal and non-metal and make sure you note the special note below.
What is covalent bonding?
Covalent bonding are bonding between relatively small size elements, with the main principle of sharing electrons between atoms such that each of the atoms in the molecules has the electron arrangement of noble gases. The two main atoms (elements) involved in covalent bonding are always small. For example Chlorine (Cl)
How to determine the formula of an ionic compound?
To determine the chemical formulas of ionic compounds, the following two conditions must be fulfilled: 1 The cation and the anion should follow the octet rule for maximum stability. 2 Ions should combine in a way that the charges of the ions must balance out and the overall ionic compound is neutral.
Why are ionic compounds brittle?
Ionic compounds are brittle – When an external force is applied to the crystals of an ionic compound, it shatters into pieces. This happens because, in the crystals of sodium chloride, the Na + ions and Cl – ions are lined up against each other in a lattice with a strong electrostatic force of attraction.
What is the term for the transfer of electrons from an electronegative element to an atom of an electronegative element
The chemical bond that is formed between 2 atoms through the transfer of one or more electrons from the electropositive or metallic element to the atom of an electronegative or non-metallic element is called an ionic or electrovalent bond .
How are the constituent ions of an ionic compound held together?
In any crystal, the constituent ions of the ionic compound are held together by electrostatic forces of attraction. The stronger the forces of attraction, the higher is the lattice energy and the more stable is the compound. This electrostatic force of attraction is determined by Coulomb’s Law.
Which rule should the cation and anion follow?
The cation and the anion should follow the octet rule for maximum stability. Ions should combine in a way that the charges of the ions must balance out and the overall ionic compound is neutral. The charges present on the anion and cation correspond to the number of electrons donated or received.
Is NaCl monovalent or ionic?
Electrovalency of sodium and chlorine in NaCl is one. Hence, they are monovalent.
What is the ionization energy of sodium?
Consider the formation of sodium ion (Na +) from sodium atom. The ionization energy of sodium is about 500kJ / mol, which is quite low. It can easily lose electrons and get converted into a sodium ion. This ion can further take part in ionic bond formation with other anions such as Cl –, Br –.