How many valence electrons does carbon need to become stable?
The electron configuration of carbon shows that the last shell of carbon has four electrons(2s 2 2p 2). Therefore, the valence electrons of carbon are …
Which elements have 5 valence electrons?
Apr 23, 2020 · Carbon is a nonmetal in group 14 of the periodic table. Like other group 14 elements, carbon has four valence electrons. Valence electrons are the electrons in the outer energy level of an atom that are involved in chemical bonds. Click to see full answer. Furthermore, how many valence electrons does a carbon atom have? four valence electrons
How do you find the number of valence electrons?
The carbon atom has four valence (outermost) electrons. Because each carbon is identical, they all have four valence electrons, so they can easily bond with other carbon atoms to form long chains or rings. How many valence electrons are in each oxygen atom? Valence electrons are the electrons in the outermost shell, or energy level, of an atom.
How many valence electrons do all the noble gases have?
Jan 25, 2020 · Carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. When it bonds only with …
Does carbon have 4 or 8 valence electrons?
Carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. When it bonds only with hydrogen, it forms compounds called hydrocarbons. Carbon can form single, double, or triple covalent bonds with other carbon atoms.20 Oct 2020
Does carbon have 1 valence electrons?
We know that the valence electrons in carbon are four. This valence electron participates in the formation of bonds with atoms of other elements. Carbon atoms form bonds by sharing electrons with hydrogen atoms. The electron configuration of hydrogen shows that hydrogen has only one electron.
Does carbon have 4 valence electrons?
Atomic carbon has six electrons: two inner shell (core) electrons in the 1s orbital, and four valence (outer most shell) electrons in the 2s and 2p orbitals.
Does carbon have 2 valence electrons?
Carbon has four valence electrons and here a valence of four.
How many electrons does carbon have?
Carbon has atomic number 6 which belongs to group 14 in the periodic table. A carbon atom has a total of 6 electrons revolving around the nucleus. The innermost shell of the carbon is fully occupied with 2 electrons in the 1s orbital while the next shell which is also the outermost shell gets partially filled with the remaining 4 electrons.
What are valence electrons?
Valence electrons are the electrons present in the outermost shell of an atom. These valence electrons actively participate in the formation of various chemical bonds if the outermost shell partially occupied. The presence of the valence electrons is responsible for the chemical properties of that element. In the case of the carbon atom, there are ...
What is the electron configuration of carbon?
So based on the Aufbau principle, the electron configuration of carbon is 1s 2 2s 2 2p 2.
Which atoms form a covalent bond?
The four valence electrons of the carbon atom form a covalent bond with the one valence electron present in the hydrogen atom.
How many valence electrons does carbon have?
Carbon has four valence electrons to achieve a whole outer energy level by forming four covalent bonds.
How to find the number of valence electrons in a carbon atom?
To determine the number of valence electrons in a carbon atom, obey these four basic steps: The first step is to determine the atomic number. The periodic table will be used to determine the atomic number of carbon. We can see that carbon has an atomic number of 6 by using the periodic table. Since the atomic number is 6, it contains six protons.
What is the valency of carbon?
As a result, carbon has a valency of four (tetravalency). The valency/oxidation states of carbon are +4 and -4. It will have a valency of +4 if it sacrifices three electrons to enter a stable condition (He). But, as previously said, if it gains ve electrons to join a steady-state (i.e., Ne), its Valency would be -4.
What is the cumulative number of electrons in an atom's outermost shell called?
Valency. The cumulative number of electrons in an atom’s outermost shell is referred to as valence electrons (i.e., in outer orbital). The degree of valence neutral atom’s electrons are still fixed; they can’t be changed (more or less) under some state within that atom, and they may or may not be shared.
How many electrons are in a carbon cell?
In other words, the carbon cell contains four electrons. To get more clearer picture to understand the difference between Valence Electrons and Valency. The cumulative number of electrons in an atom’s outermost shell is referred to as valence electrons (i.e., in outer orbital).
What are the allotropes of carbon?
Carbon’s most well-known allotropes include graphite, diamond, and buckminsterfullerene. A valence electron is an electron from an atom’s outer shell that will form a chemical bond if the outer shell is not closed; in a single covalent bond, all atoms add one valence electron to create a mutual pair. The number of c valence electrons found in the ...
How many protons does carbon have?
We can see that carbon has an atomic number of 6 by using the periodic table. Since the atomic number is 6, it contains six protons. The number of protons in neutral carbon is often equivalent to the number of electrons, implying that it has six electrons in its nucleus. Next is Compose the Electron Configuration.
Electron configuration of carbon (C) through orbit
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit (shell). These orbits are expressed by n. [n = 1,2,3,4 .
Electron configuration of carbon (C) atom through orbital
Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by ‘l’. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, f.
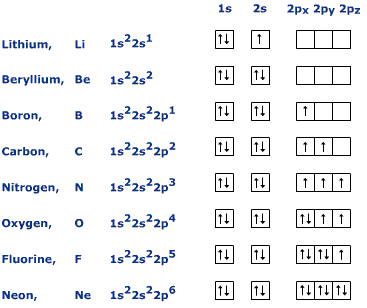
How to write the orbital diagram for carbon (C)?
To create an orbital diagram of an atom, you first need to know Hund’s principle and Pauli’s exclusion principle. Hund’s principle is that electrons in different orbitals with the same energy would be positioned in such a way that they could be in the unpaired state of maximum number and the spin of the unpaired electrons will be one-way.
Carbon (C) excited state electron configuration
Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of carbon is 1s 2 2s 2 2p 2. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Each sub-orbital can have a maximum of two electrons.
Determination of group and period
The last orbit of an element is the period of that element. The electron configuration of the carbon atom shows that the last orbit of the carbon atom is 2. So, the period of carbon is 2. On the other hand, the number of electrons present in the last orbit of an element is the number of groups in that element.
Determining the block of carbon by electron configuration
The elements in the periodic table are divided into four blocks based on the electron configuration of the element. The block of elements is determined based on the electron configuration of the element. If the last electron enters the p-orbital after the electron configuration of the element, then that element is called the p-block element.
Activation sequence of carbon atom
The elements of group-14 are relatively less active. However, the activity of the element increases as it moves from the top to the bottom of the group. Carbon is the first element of group-14. Carbon is the number one element in group-14. For this, the activation of carbon atoms is very low.