What type of bond is found in CH4?
The structure of the methane, CH4, molecule exhibits single covalent bonds. Covalent bonding involves the sharing of electrons. In the methane molecule, the four hydrogen atom share one electron each with the carbon atom. Also Know, what type of bond is found in ch4? covalent bonds
How many dozen covalent bonds are in 8G CH4?
So number of covalent bonds in 8 g CH4 = 3.011× 10^23 × 4 = 1.204 × 10^24 Well, how many DOZEN covalent bonds in 6 methane molecules?
Is C H 4 a covalent compound?
A covalent compound is described as one which is made when two or more nonmetal atoms bond by sharing valence electrons. A covalent bond is the sharing of valence electrons, and both carbon and hydrogen are nonmetals, therefore we may classify C H 4 as a covalent compound.
What is the shape of the angle of CH4?
Basically the strongest is between lone pairs, then lone pair - bonded pair then lastly bonded pair-bonded pair. CH4 has four covalent bonds and no lone pairs giving it a tetrahedral shape. The angle is therefore 109.5°.
Does CH4 have a single covalent bond?
Methane (CH 4start text, end text, start subscript, 4, end subscript) is an example of a compound where non-polar covalent bonds are formed between two different atoms. One carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (H) atom.
Does CH4 have single or double bonds?
The methane, CH4, molecule composition shows single covalent bonds. Covalent bonding entails electrons being exchanged. The four hydrogen atoms share one electron each with the carbon atom in the methane molecule.
How many bonds are present in two molecules of CH4?
Conclusion. The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each.
How many CH bonds are in CH4?
If the orbital configuration of carbon is 2s22p2 , then how can we use this information to figure out what the arrangement of the orbitals are in a simple organic molecule like methane (CH4)? It turns out that methane is tetrahedral, with 4 equal bond angles of 109.5° and 4 equal bond lengths, and no dipole moment.
How many lone pairs are in CH4?
no lone pairsThere are four bonded groups, therefore there are no lone pairs.
What type of bonds are in CH4?
Methane has four covalent bonds between carbon (C) and hydrogen (H).
How many covalent bonds are there in CH4 and c2h6?
D. 9 covalent bonds. Hint: Alkanes are the saturated hydrocarbon which is also known as paraffin. A covalent bond is a bond in which the sharing of a pair of electrons takes place between two atoms.
How do you find the number of covalent bonds?
The number of bonds for neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus the number of valence electrons. This method works because each covalent bond that an atom forms adds another electron to an atoms valence shell without changing its charge.
How is CH4 bond formed?
1:222:33Type of Bonds for CH4 (Methane) - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we'll put two electrons between atoms to form our covalent bond we have 2 four so we're formingMoreSo we'll put two electrons between atoms to form our covalent bond we have 2 four so we're forming that covalent bond.
Why is CH4 a covalent bond?
Methane, CH4, is a covalent compound with exactly 5 atoms that are linked by covalent bonds. We draw this covalent bonding as a Lewis structure (see diagram). The lines, or sticks, as we say, represent the covalent bonds. There are four bonds from a central carbon (C) linking or bonding it to four hydrogen atoms (H).
How many atoms are in a molecule of CH4?
Methane (US: MEH-thayn, UK: MEE-thayn) is a chemical compound with the chemical formula CH4 (one carbon atom bonded to four hydrogen atoms).
How many covalent bonds are there in CH4?
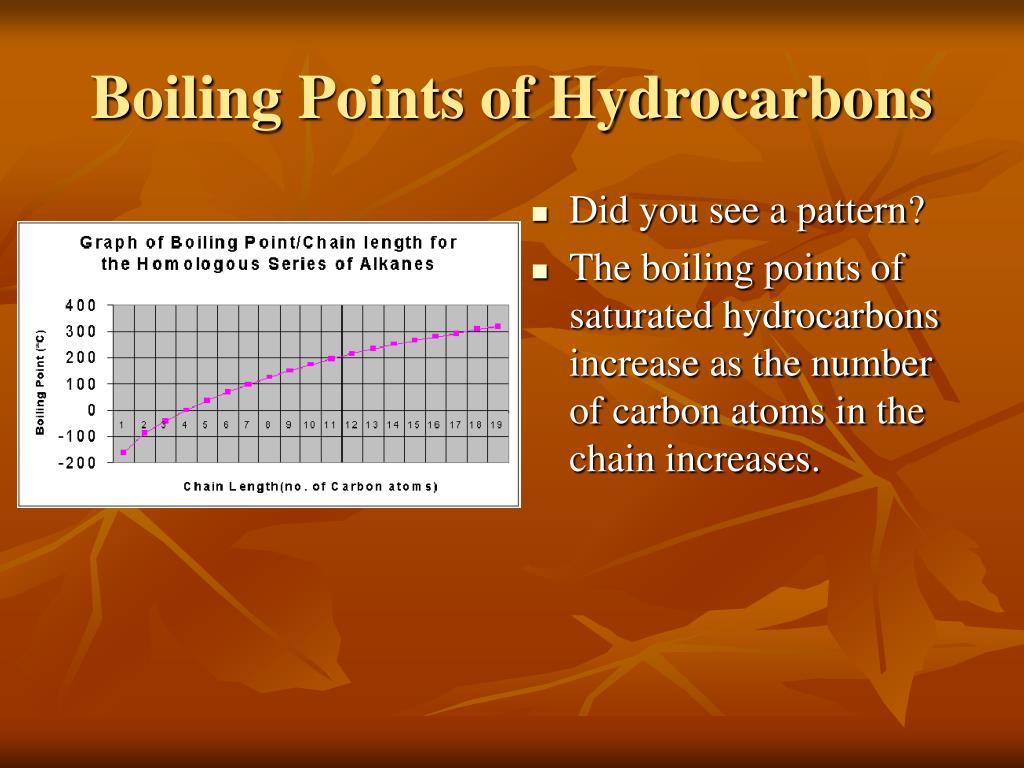
In a methane molecule (CH4) there are 4 single covalent bonds. In an octane molecule (C8H18) there are 25 single covalent bonds. How does the number of bonds affect the dispersion forces in samples of methane and octane? Which compound is a gas at room temperature? which is a liquid?
Where is the hydrogen atom in CH4?
In the methane molecule, CH4, each hydrogen atom is at a corner of a regular tetrahedron with the carbon atom at the center. In coordinates for which one of the C-H bonds is in the direction of i + j + k, an adjacent C-H bond is.
What are the two conditions necessary for a molecule to be polar?
What two conditions necessary for molecules to be polar? Her's my answer pls check. First, at least one covalent bond in the molecule has to be a polar covalent bond. For a bond to be polar, there must be a difference in the