chlorine-37. Noun. (uncountable) (physics) The minor stable isotope of chlorine, 37 17Cl, having seventeen protons and twenty neutrons; it amounts to about 24% of the element in nature. What is the number of electrons in chlorine?
| Electrons | Protons | |
|---|---|---|
| Carbon atom, 12Ca | 6 | 6 |
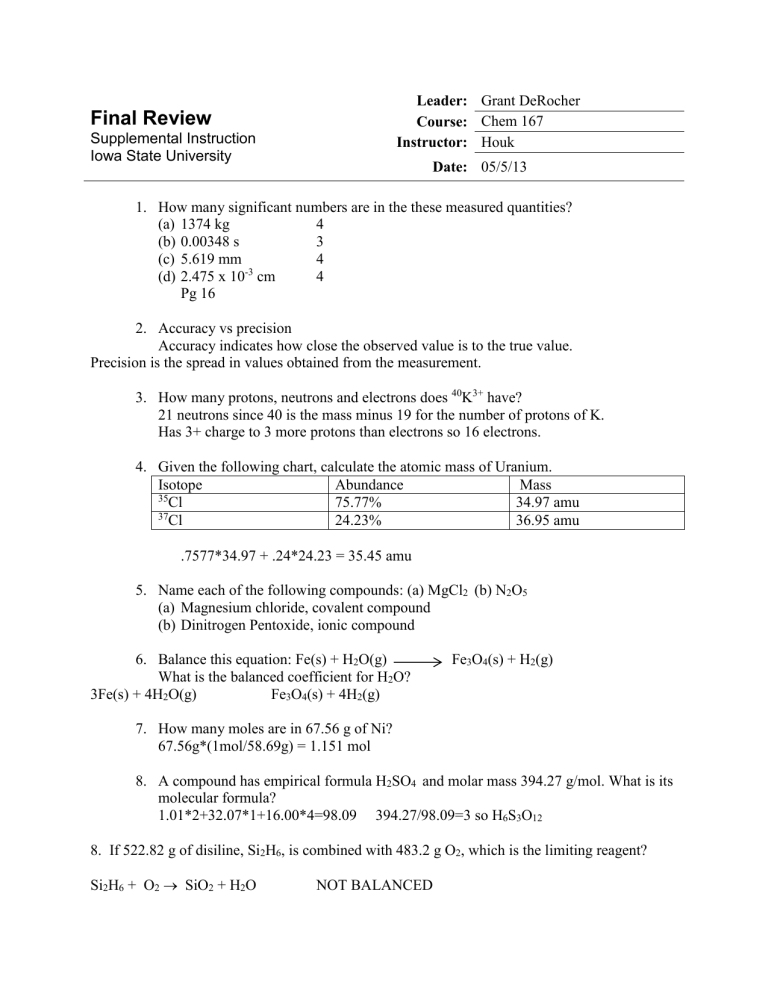
| Oxygen atom, 16O | 8 | 8 |
| Chlorine atom, 35Cl | 17 | 17 |
| Chlorine atom, 37Cl | 17 | 17 |
How many protons neutrons and electrons are in CL 37?
Feb 15, 2020 · chlorine-37. Noun. (uncountable) (physics) The minor stable isotope of chlorine, 37 17Cl, having seventeen protons and twenty neutrons; it amounts to about 24% of …
How many protons and neutrons does chlorine have?
Feb 08, 2022 · What does CL 37 mean? chlorine-37. Noun. (uncountable) (physics) The minor stable isotope of chlorine, 37 17Cl, having seventeen protons and twenty neutrons; it amounts to about 24% of the element in nature. What is the number of electrons in chlorine? 17 Which element has the atomic number 9? Fluorine How many neutrons does nitrogen have? 7
What is the half-life of each isotope of chlorine?
What does CL 37 mean? chlorine-37. Noun. (uncountable) (physics) The minor stable isotope of chlorine, 37 17Cl, having seventeen protons and twenty neutrons; it amounts to about 24% of the element in nature.
What is the difference between chlorine 36 and 37?
Sep 26, 2010 · The total nucleons is calculated by adding the amount of protons and neutrons, so if we know there are 17 protons, 37-17= 20 neutrons. There …
How many protons electrons and neutrons are in chlorine 37?
Its nucleus contains 17 protons and 20 neutrons for a total of 37 nucleons....Chlorine-37.GeneralProtons (Z)17Neutrons (N)20Nuclide dataNatural abundance24.23%4 more rows
How many neutrons are there in chlorine 37?
20 neutronsAtoms and isotopes So although chlorine has a mass number of 35 which means it has 18 neutrons, it can also have a mass number of 37, which means it has 20 neutrons.
How many electrons does an isotope of chlorine 37 have?
For example, every chlorine atom has 17 electrons and 17 protons; its atomic number is 17. However, three out of four chlorine atoms weigh 35 amu (17 protons and 18 neutrons) and the fourth weighs 37 amu (17 protons and 20 neutrons). These are the isotopes of chlorine.
How many protons does CL 37 have?
17 protonsAn atom of chlorine-35 contains 18 neutrons (17 protons + 18 neutrons = 35 particles in the nucleus) while an atom of chlorine-37 contains 20 neutrons (17 protons + 20 neutrons = 37 particles in the nucleus). Adding or removing a neutron from an atom's nucleus creates isotopes of a particular element.
What is the mass of CL 37?
"The atomic mass of chlorine-35 is 34.97u, and the atomic mass of chlorine-37 is 36.97u.Dec 10, 2020
How many protons neutrons and electrons are in an atom of chlorine-37 chlorine has an atomic number of 17?
In a neutral Cl atom, there are 17 Protons and electrons. Subtracting the atomic number from the mass number gives us 20 neutrons (37-17=20).Aug 27, 2019
How many protons does CL have?
17Chlorine / Atomic number
What is the mass number of isotopes of chlorine?
Mass numbers of typical isotopes of Chlorine are 35; 37.
How many electrons are in a neutral atom of chlorine?
Therefore, the number of electrons in neutral atom of Chlorine is 17. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
What is the electron configuration of chlorine?
Electron configuration of Chlorine is [Ne] 3s2 3p5. Possible oxidation states are +1,5,7/-1. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
How many protons does chlorine have?
Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
Why do neutrons stabilize the nucleus?
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus.
What happens when there are too many neutrons in a nucleus?
If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay . Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture.
What is the total electrical charge of the nucleus?
The total electrical charge of the nucleus is therefore +Ze , where e (elementary charge) equals to 1,602 x 10-19 coulombs. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A.