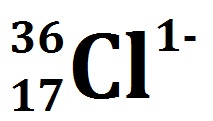Atomic Number – Protons, Electrons and Neutrons in Rhodium Rhodium is a chemical element with atomic number 45 which means there are 45 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.
How many neutrons are in rhodium?
Secondly, how many neutrons are in rhodium? 58 neutrons . Also asked, which element has a mass of 58? Cerium . What is the mass number of an isotope of copper with 34 neutrons? 63 . 39 Related Question Answers Found Why rhodium is so expensive? There are many reasons for Rhodium being so expensive. Another reason for Rhodium's price is because ...
Are protons and neutrons have the same number?
The number of protons in the nucleus of every atom of an element is always the same, but this is not the case with the number of neutrons.Atoms of the same element can have a different number of neutrons.Atoms want to have the same number of neutrons and protons but the number of neutrons can change.
What number represents the protons and nuetrons?
- Because an electron has a negative charge, when you remove electrons, the ion becomes positive. When you add more electrons, the ion becomes negative.
- For example, N 3- has a -3 charge while Ca 2+ has a +2 charge.
- Keep in mind that you do not have to do this calculation if there is no superscripted ion number following the element.
How many neutrons and protons are in rutherfordium?
The number 104 is the element's atomic number, which tells you the number of protons in the nucleus. Subtracting that number from the stable isotope (267-104) gives you the number of neutrons, or 163.
What is the neutron of Rhodium?
SummaryElementRhodiumNumber of protons45Number of neutrons (typical isotopes)103Number of electrons45Electron configuration[Kr] 4d8 5s11 more row
How many protons are in a Rhodium atom?
45Rhodium / Atomic number
How many electrons are in an atom of Rhodium?
45 electronsRhodium atoms have 45 electrons and the shell structure is 2.8. 18.16. 1.
What has 45 protons and 59 neutrons?
Rhodium (Rh) - Atomic Number 45.
How many valence electrons does rhodium?
How many valence electrons does rhodium have? Ans: Nine valence electrons.
How many protons does rhodium-103 have?
Rhodium (Rh). Diagram of the nuclear composition and electron configuration of an atom of rhodium-103 (atomic number: 45), the most common isotope of this element. The nucleus consists of 45 protons (red) and 58 neutrons (blue).
What element has 3 protons 4 neutrons and 2 electrons?
A lithium atom contains 3 protons in its nucleus irrespective of the number of neutrons or electrons.
What is the electron configuration of rhodium?
Kr 4d8 5s1Rhodium / Electron configuration
What element has 2 protons?
The element of an atom with 2 protons is always helium. If you are given the atomic weight of an atom, you need to subtract the number of neutrons to get the number of protons. Sometimes you can tell the elemental identity of a sample if all you have is the atomic weight.
How many protons does a hydrogen atom have?
It's easy to get a hydrogen atom with one proton and one neutron (deuterium), yet you won't find a helium atom with an atomic weight of 2 because this would mean the helium atom had two protons and zero neutrons! If the atomic weight is 4.001, you can be confident the atom is helium, with 2 protons and 2 neutrons.
How to find the number of neutrons in an atom?
You can find the number of neutrons if you know the isotope of the atom. Simply subtract the number of protons (the atomic number) from the mass number to find the remaining neutrons.
Why is the periodic table decimal?
Each atom has an integer number of neutrons, but the periodic table gives a decimal value because it is a weighted average of the number of neutrons in the isotopes of each element. So, what you need to do is round the atomic weight to the nearest whole number to get a mass number for your calculations.
What does it mean when an ion has a 2+ charge?
If an ion has a 2+ charge, like Zn 2+, this means there are two more protons than electrons. If the ion has a 1- charge (simply written with a minus superscript), then there are more electrons than the number of protons. For F -, the number of protons (from the periodic table) is 9 and the number of electrons is:
How to tell if an atom is neutral or positive?
For a neutral atom, the number of electrons is the same as the number of protons. Often, the number of protons and electrons is not the same, so the atom carries a net positive or negative charge. You can determine the number of electrons in an ion if you know its charge. A cation carries a positive charge and has more protons than electrons.
Which atom has more protons than electrons?
A cation carries a positive charge and has more protons than electrons. An anion carries a negative charge and has more electrons than protons. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. The number of protons of an atom cannot change via any chemical reaction, ...