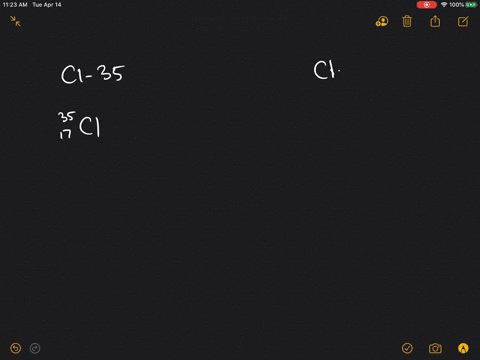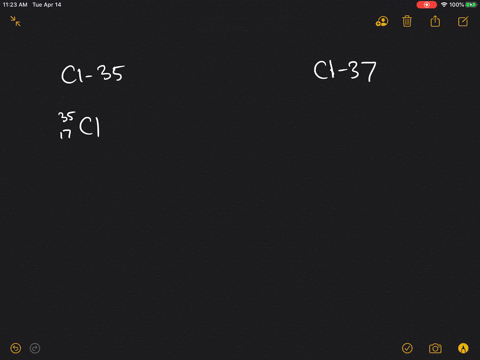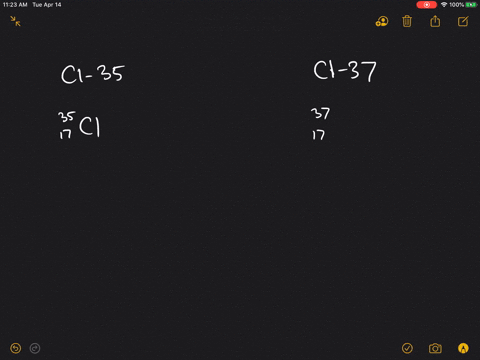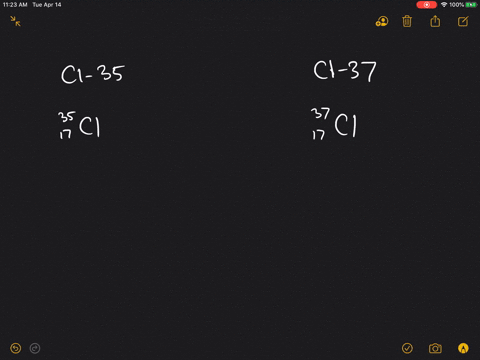| Electrons | Neutrons | |
|---|---|---|
| Chlorine atom, 35Cl | 17 | 18 |
| Chlorine atom, 37Cl | 17 | 20 |
| Naturally occurring mixture of chlorine | 17 | 18 or 20 |
| Uranium atom, 234U | 92 | 142 |
How many protons and neutrons should be in the isotope chlorine-35?
An atom of chlorine-35 contains 18 neutrons (17 protons + 18 neutrons = 35 particles in the nucleus) while an atom of chlorine-37 contains 20 neutrons (17 protons + 20 neutrons = 37 particles in the nucleus). Adding or removing a neutron from an atom's nucleus creates isotopes of a particular element.
How many electrons does the isotope chlorine-35 have?
How many protons electrons and neutrons are in the chlorine 35 isotope?NameChlorineAtomic Mass35.453 atomic mass unitsNumber of Protons17Number of Neutrons18Number of Electrons17Apr 16, 2020
How many neutrons does isotope chlorine-35 have?
18 neutronsSome atoms of chlorine have 18 neutrons in their nuclei. Other atoms of chlorine may have 20 neutrons in the nucleus. These atoms of chlorine have different atomic mass numbers. The isotope that has 18 neutrons has an atomic mass number of 35.
How many protons neutrons and electrons are there in chlorine 36?
Chlorine 36 (Cl-36) is the chlorine isotope whose nucleus consists of 17 protons and 19 neutrons. It is a cosmogenic radioisotope of chlorine with a trace half-life of 301,000 years in the environment, in a ratio of about 7 × 10-13 to 1 with stable isotopes.
How many protons does chlorine have?
17Chlorine / Atomic number
How many protons and electrons does chlorine have?
Although every atom has equal numbers of protons and electrons, the number of neutrons can vary. For example, every chlorine atom has 17 electrons and 17 protons; its atomic number is 17.
How many protons are in chlorine isotopes?
17 protonsIts nucleus contains 17 protons and 20 neutrons for a total of 37 nucleons....Chlorine-37.GeneralProtons (Z)17Neutrons (N)20Nuclide dataNatural abundance24.23%4 more rows
How do you find protons?
The number of protons in the nucleus of the atom is equal to the atomic number (Z). The number of electrons in a neutral atom is equal to the number of protons. The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus.
How many protons are in an chlorine atom with a mass number of 35?
The mass number (i.e., the total number of protons and neutrons) of chlorine-35 is 35. The atomic number (i.e., the number of protons) of chlorine is 17.
What is the mass number of chlorine 35?
34.968852Reference E95IsotopeMassSpin35Cl34.9688523/237Cl36.9659033/22 more rows
How do you find the electrons of chlorine?
3:024:09How to find the Number of Protons, Electrons, Neutrons for Chlorine (Cl)YouTubeStart of suggested clipEnd of suggested clipWe would put the mass number and the atomic number is the same because it's chlorine all chlorineMoreWe would put the mass number and the atomic number is the same because it's chlorine all chlorine has an atomic number of 17 17 protons so this is nuclear notation for isotopes.
How do you find the protons neutrons and electrons of chlorine?
So in chlorine, we can see that the atomic number is 17. This means that each chlorine atom has 17 protons and therefore must also have 17 electrons. The nucleus of an atom contains protons and neutrons.
How many protons neutrons and electrons does chlorine-35 have?
An atom of chlorine-35 contains 18 neutrons ( 17 protons + 18 neutrons = 35 particles in the nucleus) while an atom of chlorine-37 contains 20 neutrons (17 protons + 20 neutrons = 37 particles in the nucleus). Adding or removing a neutron from an atom’s nucleus creates isotopes of a particular element.
How many electrons does the isotope chlorine-35 have?
Hence, the number of protons in chlorine-35 is equal to the number of electrons in chlorine-35. So, the number of electrons in chlorine-35 is 18.
How many protons and neutrons should be in the isotope chlorine-35?
Chlorine has two common isotopes, Chlorine-35 and Chlorine-37. Chlorine-35 has 17 protons and 18 neutrons and occurs in nature about 75% of the time.
How many protons electrons and neutrons are in the chlorine-35 isotope quizlet?
The atomic number of chlorine is 17. Calculate the numbers of Protons, electrons, and neutrons each isotope has. Both isotopes have 17 protons and 17 electrons. Chlorine-35 has 18 neutron and chlorine-37 has 20 neutrons.
How many protons and electrons does chlorine have?
Chlorine has an atomic number of 17 and an atomic mass of 35.45, meaning that an atom of chlorine consists of 17 protons, 17 electrons, and 18 neutrons.
How many protons does chlorine 35 have?
An element with 17 protons will always be chlorine. However an element’s mass numbers can vary, which means that it can have different numbers of neutrons. So although chlorine has a mass number of 35 which means it has 18 neutrons, it can also have a mass number of 37, which means it has 20 neutrons.
How do you find the atomic number?
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11.
How many neutrons are in chlorine 35?
An atom of chlorine-35 contains 18 neutrons (17 protons + 18 neutrons = 35 particles in the nucleus) while an atom of chlorine-37 contains 20 neutrons (17 protons + 20 neutrons = 37 particles in the nucleus). Adding or removing a neutron from an atom's nucleus creates isotopes of a particular element.
What is the abundance of chlorine 35?
The abundance of chlorine-35 is 75% and the abundance of chlorine-37 is 25%. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37. One may also ask, what is the atomic number of chlorine 35?
How many protons does chlorine have?
Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
What is the mass number of isotopes of chlorine?
Mass numbers of typical isotopes of Chlorine are 35; 37.
How many electrons are in a neutral atom of chlorine?
Therefore, the number of electrons in neutral atom of Chlorine is 17. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
What is the electron configuration of chlorine?
Electron configuration of Chlorine is [Ne] 3s2 3p5. Possible oxidation states are +1,5,7/-1. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
Why do neutrons stabilize the nucleus?
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus.
What happens when there are too many neutrons in a nucleus?
If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay . Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture.
What is the total electrical charge of the nucleus?
The total electrical charge of the nucleus is therefore +Ze , where e (elementary charge) equals to 1,602 x 10-19 coulombs. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A.
Chlorine-35 Isotope Applications
Chlorine-35 isotope is used for production of Chlorine-36 (Cl-36 isotope, 36Cl isotope) radionuclide (radioisotope) for use in e.g. biological research and life science;
Chlorine-35 Information
Chlorine is a halogen element. It is the poisonous greenish-yellow gas. This element occurs widely in nature as sodium chloride in seawater. It reacts directly with many elements and compounds, strong oxidizing agent. Chlorine was discovered by Karl Scheele in 1774. Humphrey David confirmed chlorine as an element in 1810.