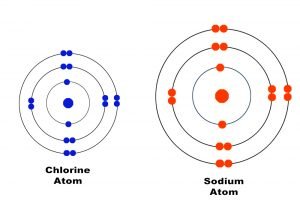
How many electrons are shared by chlorine?
There are two electrons shared by the two Cl atoms so there is a single bond between the Cl atoms. Each chlorine has 7 valence electrons plus the one it is sharing for a total of 8 valence electrons. So each Cl atom will have 8 valence electrons as a result of sharing. This is the rule of octet.
How many neutrons can be found in chlorine?
So although chlorine has a mass number of 35 which means it has 18 neutrons, it can also have a mass number of 37, which means it has 20 neutrons. The different types of chlorine are called isotopes of chlorine. Isotopes are forms of an element that have the same number of protons but different numbers of neutrons.
What gas has 48 neutrons?
The fourth noble gas of atomic number 36 and symbol Kr is known as Krypton.It contains 36 protons and electrons each and 48 neutrons. Krypton with atomic mass 83.798 has a set of isotopes in large number. It was derived from the Greek word ‘Kryptos’ which mean hidden.
What are facts about chlorine?
When your doctor examines you for signs of chlorine poisoning, they look for:
- Fast breathing
- Blue skin
- Rapid heartbeat
- Wheezing
- Your stomach sinking in under your ribs when breathing
- Nasal flaring when breathing
- High pitched sound when breathing
- Hemorrhage (outflow of blood) in your respiratory tract
- Runny nose
- Excessive salivation
How many electrons are in a neutral atom of chlorine?
Therefore, the number of electrons in neutral atom of Chlorine is 17. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
How many protons does chlorine have?
Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
What is the electron configuration of chlorine?
Electron configuration of Chlorine is [Ne] 3s2 3p5. Possible oxidation states are +1,5,7/-1. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
Why do neutrons stabilize the nucleus?
Neutrons stabilize the nucleus, because they attract each other and protons , which helps offset the electrical repulsion between protons. As a result, as the number of protons increases, an increasing ratio of neutrons to protons is needed to form a stable nucleus.
What happens when there are too many neutrons in a nucleus?
If there are too many or too few neutrons for a given number of protons, the resulting nucleus is not stable and it undergoes radioactive decay . Unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, or electron capture.
What is the mass number of isotopes of chlorine?
Mass numbers of typical isotopes of Chlorine are 35; 37.
What is the total electrical charge of the nucleus?
The total electrical charge of the nucleus is therefore +Ze , where e (elementary charge) equals to 1,602 x 10-19 coulombs. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A.
Neutron and Mass Numbers and Nuclear Properties
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1.
Neutron and Atomic Numbers and Nuclear Stability
Nuclear stability is a concept that helps to identify the stability of an isotope. To identify the stability of an isotope it is needed to find the ratio of neutrons to protons. To determine the stability of an isotope you can use the ratio neutron/proton (N/Z).
How many neutrons are in chlorine 35?
An atom of chlorine-35 contains 18 neutrons (17 protons + 18 neutrons = 35 particles in the nucleus) while an atom of chlorine-37 contains 20 neutrons (17 protons + 20 neutrons = 37 particles in the nucleus). Adding or removing a neutron from an atom's nucleus creates isotopes of a particular element.
What is the abundance of chlorine 35?
The abundance of chlorine-35 is 75% and the abundance of chlorine-37 is 25%. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37. One may also ask, what is the atomic number of chlorine 35?