How many neutrons are found in the nucleus of copper?
Mar 06, 2020 · As atomic mass of copper is 64. Hence, calculate the number of neutrons present in a copper atom as follows. Therefore, we can conclude that in an atom of …
How many neutrons are in coppers most stable isotopes?
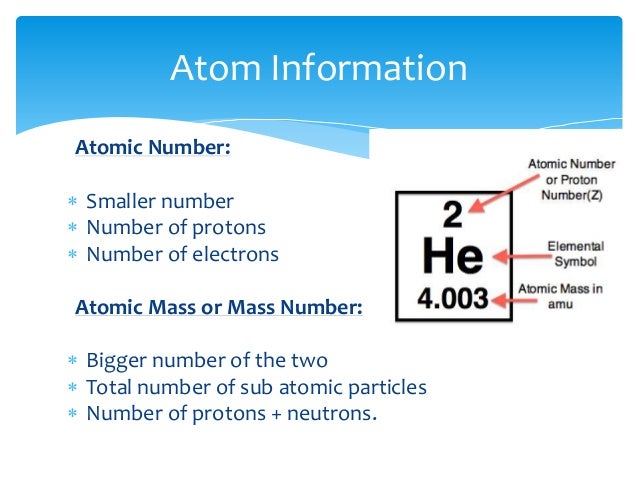
Copper has an atomic number of 29 and a mass number of 63. This means that there are 29 protons and 34 neutrons in the nucleus of the copper atom (29 + 34 = 63). Next, which atom has 34 neutrons? The copper isotope contains 34 neutrons.
What is the number of protons in copper?
How many neutrons are in an atom of copper? Beryllium has atomic number 4, which means it has 4 protons in its nucleus, and 4 electrons in its orbitals (in the configuration 1 s 2 2 s 2 ). The only stable isotope of Beryllium is 9 B e , which has 9 \u2212 4 = 5 neutrons in the nucleus.
What is the average atomic mass of copper?
7 rows · Nov 21, 2020 · Mass numbers of typical isotopes of Copper are 63; 65. The total number of neutrons in the ...
Neutron and Mass Numbers and Nuclear Properties
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1.
Neutron and Atomic Numbers and Nuclear Stability
Nuclear stability is a concept that helps to identify the stability of an isotope. To identify the stability of an isotope it is needed to find the ratio of neutrons to protons. To determine the stability of an isotope you can use the ratio neutron/proton (N/Z).
How many electrons are in a copper atom?
Therefore, the number of electrons in neutral atom of Copper is 29. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
How many protons does copper have?
Copper is a chemical element with atomic number 29 which means there are 29 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
What is electron configuration?
The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. Every solid, liquid, gas, and plasma is composed ...
What is copper used for?
The major applications of copper are electrical wire (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is used mostly as a pure metal, but when greater hardness is required, it is put into such alloys as brass and bronze (5% of total use).
Where are protons and neutrons found?
The protons exist in the nuclei of typical atoms, along with their neutral counterparts, the neutrons. Neutrons and protons, commonly called nucleons, are bound together in the atomic nucleus, where they account for 99.9 percent of the atom’s mass.
What is the total electrical charge of the nucleus?
The total electrical charge of the nucleus is therefore +Ze , where e (elementary charge) equals to 1,602 x 10-19 coulombs. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A.
What is the difference between neutrons and atomic numbers?
The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z. For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons.
Answer
Atomic number of copper is 29. The atomic number of an atom is determined by the number of protons in the nucleus. Therefore, there are 29 protons in an atom of copper. In a neutral atom, there are equal number of protons and electrons. Therefore, there are 29 electrons in a copper atom. Mass number of copper is 64.
New questions in Physics
How can you drop two eggs the fewest amount of times, without them breaking? ...