Regarding this, how many Lewis structures does co32? There are three different possible resonance structures from carbonate. Each carbon oxygen bond can be thought of as 1.333 bonds. the average of a double bond and 2 single bonds. 4 bonds/3 structures.
Full Answer
How do you know how many bonds are in a Lewis structure?
To know the no. of σ and π bonds the Lewis structure of the compound is to be drawn showing all single,double and triple bonds. In this structure Each single bond is 1σ bond
How to write Lewis structure?
- Arrange the atoms to show specific connections. ...
- Determine the total number of valence electrons in the molecule or ion. ...
- Place a bonding pair of electrons between each pair of adjacent atoms to give a single bond. ...
- Beginning with the terminal atoms, add enough electrons to each atom to give each atom an octet (two for hydrogen). ...
What are the Lewis structure rules?
Lewis Structure Examples
- Lewis Structure of CO2 The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. ...
- Lewis Structure of O2 An atom of oxygen contains 6 electrons in the valence shell. ...
- Lewis Structure of CO (Carbon Monoxide)
What are some examples of Lewis structures?
Summary: Lewis Structures, VSEPR, and Molecular Polarity
- “Electron groups” include bonds, lone pairs, and odd (unpaired) electrons. A multiple bond (double bond or triple bond) counts as one electron group.
- A multiple bond (double bond or triple bond) counts as one bond in the VSEPR model.
- A = central atom, X = surrounding atoms, E = lone pairs
How many Lewis structures can be drawn for CO32?
three resonance structuresThe Carbonate (CO2−3) Ion Like ozone, the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. Unlike O3, though, the actual structure of CO32− is an average of three resonance structures.Nov 6, 2021
Is CO32 Lewis structure?
0:001:58How to Draw the Lewis Structure for CO3 2- (Carbonate Ion) - YouTubeYouTubeStart of suggested clipEnd of suggested clipLet's do the CEO three two - Lewis structure the carbonate ion carbon. Has four valence electronsMoreLet's do the CEO three two - Lewis structure the carbonate ion carbon. Has four valence electrons oxygen has six we have three oxygens.
How many equivalent structures does CO32?
How many equivalent resonance structures are possible for co32? There are three different possible resonance structures from carbonate. Each carbon oxygen bond can be thought of as 1.333 bonds.Jul 20, 2020
How many electrons are in CO32?
Thirty-two electrons as required.Oct 4, 2017
What shape is CO32?
trigonal planar3 that the molecular geometry of CO 3 2− is trigonal planar with bond angles of 120°.Jun 5, 2019
What are the resonance structures for co32?
0:004:57Resonance Structures of CO3(-2), the Carbonate Ion - YouTubeYouTubeStart of suggested clipEnd of suggested clipBetween the central atom and all the surrounding atoms that accounts for two four six electrons.MoreBetween the central atom and all the surrounding atoms that accounts for two four six electrons. Then i fill the octets on the outer. Atoms each of the oxygens.
How many single bonds does co32 have?
0:291:29Number of Lone Pairs and Bonding Pairs for CO3 2- (Carbonate ion)YouTubeStart of suggested clipEnd of suggested clipSo we have a total of one two three four pairs of bonding electrons on the central. Carbon. We don'tMoreSo we have a total of one two three four pairs of bonding electrons on the central. Carbon. We don't have any lone pairs all of these electrons they're involved in chemical bonds between the carbon
How many bonds and lone pairs does co32 have?
No. of lone pair=8 ∵ 8 pair of e− of oxygen did not participated in bonding.
How many electrons are in CO32?
In CO32- ion, we have one carbon atom and three oxygen atoms along with two negatively charged electrons carrying the charge. Valence electrons refer to the number of electrons in the outermost shell of an atom around the nucleus that help in determining the valency of the given atom.
What is Lewis structure?
Lewis Structure is the name given to such a skeletal diagram where we use the symbols of the atoms and use dots to represent the valence shell electrons. Hence, Lewis Structure is also commonly called Electron Dot Structure. Let us proceed to draw the most appropriate LS diagram of CO32- ion. Step 1: Count the Total Number of Valence Electrons.
What is the octet rule?
Octet Rule. The elements present in the main group usually tend to follow the concept of octet fulfillment. This means that these atomic elements will incline towards having eight valence electrons just like the noble gas configurations of the same period. Let us draw the skeletal diagram for CO32- ion:
Is CO32 a water soluble oxocarbon?
Some of these include glass and ceramic creation, food preservation, and iron extraction. CO32- ion is the simplest oxocarbon anion that decomposes on heat ing and is usually water-insoluble barring a few exceptions . Let us now study the chemical bonding of the CO32- ion in detail.
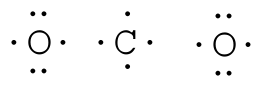
CO32- Lewis Structure
CO32- Molecular Geometry
- Is a 2D structure sufficient enough for getting an in-depth understanding of the bonding happening inside a molecule? Well, a perfectly drawn Lewis Structure does introduce to us the basic representation of constituent atoms inside any molecule or ion and also talks about the type of bonds formed. But this is not enough. Here comes the VSEPR theory or Valence Shell Electro…
CO32- Hybridization
- If you are a student of chemistry, it is safe to assume that you are aware of the difference between an orbit and an orbital. While orbit talks about the definite path of an electron around the atomic nuclei, orbital deals with the probability of electrons being present in any given space. Atomic orbitals are of different shapes like spherical and dumb-bell shapes to name a few. Acco…
CO32- Molecular Orbital (MO) Diagram
- What is MO theory?
Molecular Orbital Theory is a concept of quantum mechanics that is used to decipher the chemical bonding nature inside different molecular structures. This is a complex yet useful tool that helps in sketching MO diagrams for better understanding. This theory treats electrons to be …
Conclusion
- Here, in this detailed article, we have done an extensive discussion on the chemical bonding nature of the famous carbonate anion. We have covered the formation of Lewis Structure, deciphered the perfect molecular geometry and bond angles of 3D CO32-. Not only this, but we have also tackled orbital hybridization and quantum MO theory. Happy learning!