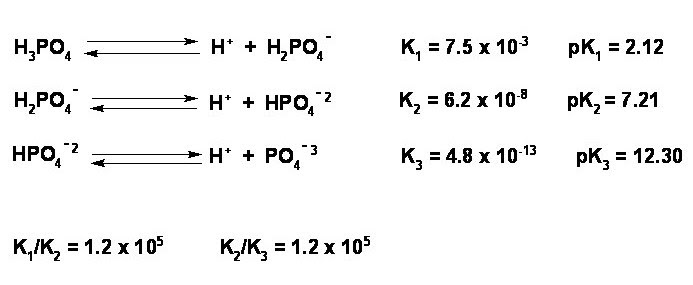What is the use of LiBr in organic chemistry?
It is also used as a salt in absorption chilling along with water (see absorption refrigerator ). Solid LiBr is a useful reagent in organic synthesis.
How to write the name of LiBr?
Get more practice naming compounds. For LiBr use the hints and resources below to help write the name. LiBr is a binary ionic compound. Name the metal (Lithium) as it appears on the Periodic Table. Name the non-metal (Sulf ur) by replacing the ur with ide.
Which would have a greater ionic character - BCl3 or CCl4?
Electronegativit of Boron is less than that of Carbon. Hence the B-Cl bond has greater electronegativity difference than C-Cl bond. So we can conclude that Bcl3 would have greater ionic character than CCl4.
What is the standard state of lithium bromide (LiBr)?
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). ?) Lithium bromide ( LiBr) is a chemical compound of lithium and bromine.
What polyatomic ions have a 3 charge?
Luckily, we know the charge on the anion: phosphate is a polyatomic ion that always has a charge of 3-.
What polyatomic ions have a 2 charge?
Common Polyatomic Ions-2 CHARGEionnameHPO32-hydrogen phosphiteHPO42-hydrogen phosphateCO32-carbonate26 more rows
What is multi atomic ion?
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. Unlike a molecule, which has a net charge of zero, this chemical species is an ion.
Which one is a polyatomic ion?
Polyatomic ions are ions which consist of more than one atom. For example, nitrate ion, NO3-, contains one nitrogen atom and three oxygen atoms.
How do you find the number of polyatomic ions?
0:432:15Oxidation numbers polyatomic ions | Ammonium sulfite (NH4)2SO3 - Dr KYouTubeStart of suggested clipEnd of suggested clipNumber for polyatomic ion is equals to the ions charge.MoreNumber for polyatomic ion is equals to the ions charge.
How do you calculate polyatomic ions?
Calculate from Oxidation Number The oxidation number of oxygen is -2, and the oxidation number of hydrogen is +1. Add together the oxidation numbers of all the atoms in the polyatomic ion. In the example, -2 +1 = -1. This is the charge on the polyatomic ion.
What are the 7 diatomic elements?
So our Mnemonic is: Have No Fear Of Ice Cold Beer. So these are our seven diatomic elements: Hydrogen, Nitrogen, Flourine, Oxygen, Iodine, Chlorine, Iodine, and Bromine.
What are ions Class 9?
An ion is a positively or negatively charged atom or group or atom. For Ex:Sodium ion,Magnesium ion,magnesium ion,chloride ion,oxide ion etc. There are two types of ions: 1)Cation:A positively charged ion is known as cation. A cation is formed by loss of one or more electrons by an atom.
What are polyatomic ions Class 9 Ncert?
Polyatomic ions: Those ions which are formed from groups of joined atoms are called polyatomic ions. Compound ions are also called polyatomic ions. For e.g. Ammonium ion NH4+, is a compound ion which is made up of two types of atoms joined together nitrogen and hydrogen.
What are all the polyatomic elements?
Terms in this set (13)Arsenic. As₂Astatine. At₂Bromine. Br₂Chlorine. Cl₂Fluorine. F₂Hydrogen. H₂Iodine. I₂Nitrogen. N₂More items...
How many atoms are there in polyatomic molecules?
Polyatomic molecules are electrically neutral groups of three or more atoms held together by covalent bonds.
Is Na+ a polyatomic ion?
compounds, except that we use the name of the polyatomic ion whenever it occurs. For example, NaNO2 is named according to its cation, Na+ (sodium), and its polyatomic anion, N03 (nitrite). Its full name is sodium nitrite.
What is the mass of 2.6 moles of LiBr?
So the molar mass (mass of 6.02214076x10^23 of anything (in this case molecules))of LiBr is (6.941+79.90=86.841 g/mol, g/mol=grams per mole, 6.941= (Li) atomic mass, 79.90= (Br) atomic mass)86.841 g/mol, so 2.6 moles of LiBr would have a mass of about (86.841x2.6=225.7866)225.7866 grams.
Which group of the periodic table is lithium in?
Lithium resides in group 1 of the periodic table and Bromine in group 7.
How to find the ionic character of a compound?
Ionic character can be determined by comparing the electronegativity (EN) of the two elements in each compound. The greater the difference in electronegativity between the two elements, the greater the ionic character. So, for LiF, look up the EN for Li and F, then subtract the smaller EN (ie; Li) from the larger EN (ie; F)….
How to determine ionic or covalent nature of a compound?
One way to determine the ionic/covalent nature of a compound is by comparing the Polarizability of the anions. Polarizability (tendency of an anion to get distorted/polarized) of an anion increases with the increase in size of the anion. Thus, the Polarization and hence the Covalence increases with size of anion.
Why is Li+ smaller than Na+?
Now Li+ is only a bit smaller than Na+ because Na" has a larger core charge, compressing its greater number of electrons more tightly -- actually the greater number of repelling electrons in this last occupied L-shell of Na+ repel one another. Anyway this greater repulsion between this greater number of electrons wins out over the bigger core charge.
How to find the difference in electronegativity?
So, for LiF, look up the EN for Li and F, then subtract the smaller EN (ie; Li) from the larger EN (ie; F)… (EN F - EN Li). This gives you a numerical value called the difference in electronegativity (dEN). Do the same for each of the other compounds and compare. You can the rank them in order of increasing ionic character (lowest dEN to highest dEN).
Why is NaF ionic?
Answer is NaF because for ionic bond more the metallic and non metallic character more is the ionic character.
Which metal has the highest period alkaline?
So rather than Li, we should choose the highest period alkaline metal. That is Francium , but Francium is radioactive and has a very short half life (22 min for the most stable isotope, thank you Wikipedia), so the answer you are looking for is probably CsF. Another way to settle this is to look at a periodic table that list the electronegativity of each element, and the pick the one that has the highest and the one with the lowest. However, this would also be an approximation, to know for certain you would need a quantum computing calculation.
Which is larger, iodine or chloride?
Iodide ion is a LARGER, and LESS CHARGE-DENSE anion than chloride ion. Why? Because iodine is a 5th row non-metal, and chloride a 3rd row non-metal..
Why is lithium chloride used in lab animals?
Lithium chloride is used as an aversive agent in lab animals to study conditioned place preference and aversion .
Is lithium a compound or a compound?
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characters) , although the small size of the Li + ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents (83.05 g/100 mL of water at 20 °C) and its hygroscopic properties.
Is lithium chloride an anhydrous salt?
Mono-, tri-, and pentahydrates are known. The anhydrous salt can be regenerated by heating the hydrates. LiCl also absorbs up to four equivalents of ammonia /mol. As with any other ionic chloride, solutions of lithium chloride can serve as a source of chloride ion, e.g., forming a precipitate upon treatment with silver nitrate :