How many atoms are in Al2 (SO4) 3?
The simple answer is that there are 51 atoms and 15 ions in three times the chemical formula for aluminium sulfate. In the chemical formula Al2 (SO4)3, the Al2 means there two aluminium (atoms or ions). The SO4 is a sulfate ion and (SO4)3 means there are 3 sulfate ions. Click to see full answer.
How many sulfate ions are in SO4 3?
The SO4 is a sulfate ion and (SO4)3 means there are 3 sulfate ions. Click to see full answer. Similarly, it is asked, how many atoms are in the compound al2 so4 3?
How many atoms are there in 3x2 aluminium sulfate?
There are 6 aluminium ions (3x2) and 9 sulfate ions (3x3). Now to work out the number atoms. That depends exactly how you define an atom, but let’s keep things simple at first. The aluminium is present as ions, but you would normally count them as “atoms”, when counting the number of atoms.
How many atoms are in a sulfate ion?
Therefore, the simple answer is that there are a total of 51 atoms, comprising 6 aluminium atoms, 9 sulfur atoms and 36 oxygen atoms. Now let’s consider some complications. Diagrams of the sulfate ion often show just two of the oxygen atoms with a negative charge.
How many ions does aluminum sulfate have?
The ionic compound must be electrically neutral. Thus we take 2 aluminum ions (6+ charge) and 3 sulfate ions (6-) charge, and achieve electrical neutrality.
Which type of ions are present in Aluminium sulphate?
Aluminium sulfate is an ionic compound, a combination of both positive and negative ions. Ions are charged atoms, which may either be monatomic ions (single atoms) or polyatomic (multiple atoms combined to form a charged part). Aluminium forms a + 3 ion, Al+3, and sulphate is the -2 polyatomic ion, (SO4)-2.
How do you find the number of ions in a compound?
Number of mol is calculated by ratio of given mass to the molar mass. After calculating the number of moles, the number of ions will be equal to the product of the number of moles and Avogadro's number.
How many ions are in Al2 co3 3?
0:152:07Is Al2(CO3)3 (Aluminum carbonate) Ionic or Covalent/Molecular?YouTubeStart of suggested clipEnd of suggested clipSometimes called 3a it has an ionic charge of 3 plus so that 3 plus each of these aluminums willMoreSometimes called 3a it has an ionic charge of 3 plus so that 3 plus each of these aluminums will have a charge of 3.
How do you find ions?
2:296:02How to find ions in a compound | Dissociation of solutions - Dr KYouTubeStart of suggested clipEnd of suggested clipWith some positive charge and the anion is going to be F minus. So we'll figure out the actualMoreWith some positive charge and the anion is going to be F minus. So we'll figure out the actual charge snacks. And we're going to use cross over method for it. So one from al will cross over to F.
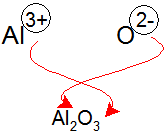
How many ions are in Al2O3?
If we combine two Al3+ ions and three O2- ions, and use subscripts to indicate the number of each ion in the formula, we obtain Al2O3 as the empirical formula. The easiest method to determine the formula for an ionic compound, when given the component ions, is to use the criss-cross method.
How many ions are there?
There are two types of ions : cations. anions.
How many ions are in cacl2?
two chloride ionsThe molecule for calcium chloride has one calcium ion (+2) and two chloride ions (-1), which means that the overall charge for the molecule is 0, or neutral.
How many molecular ions of SO4 are needed for 2 atoms of Al2?
The answer is so simple, we’ll need 3 molecular ions of SO4 , as it’s obvious by the chemical formula of Al2 (SO4)3. So 3 molecular ions of SO4 for 2 atoms of Al, means 1.5 Avogadro’s number molecular ions of SO4 for 1 Avogadro’s number atoms of Al that is 9.03 * 10^23 molecular ions of SO4.
How many atoms are in Al2?
The simple answer is that there are 51 atoms and 15 ions in three times the chemical formula for aluminium sulfate. In the chemical formula Al2 (SO4)3, the Al2 means there two aluminium (atoms or ions).
How many atoms are in an al2 sulfate molecule?
As you can see from the formula, in each aluminium sulfate ( Al 2 ( SO 4) 3) molecule, there are 2 aluminium ( Al) atoms , 3 sulfur ( S) atoms and 12 oxygen ( O) atoms. 2 aluminium atoms and 12 oxygen atoms. That means that the ratio of aluminium atoms and oxygen atoms is 1:6.
How many atoms are in aluminium sulfate?
In 1 molecule of aluminium sulfate, there are 2 aluminium atoms and 3 sulfate anions, each containing 1 sulfur atom and 4 oxygen atoms. 2 aluminium atoms, 3 sulfur atoms, and 12 oxygen atoms. Now, take a look at the atomic mass (Ar) of each atom in the molecule.
What is aluminum sulfate anhydrous?
Aluminum Sulfate Anhydrous is an aluminum salt with immune adjuvant activity. This agent adsorbs and precipitates protein antigens in solution; the resulting precipitate improves vaccine immunogenicity by facilitating the slow release of antigen from the vaccine depot formed at the site of inoculation.
How does alum help with deodorization?
By inhibiting or deactivating odor-producing bacteria, there is little to none metabolism of sweat components thus decreasing the occurrence of body odor. Recent studies suggest that the active binding of alum to the membranes of dendritic cells (DCs) result in alteration of lipid membranes structures as a key process in alum's adjuvant effect in vaccines. As new adjuvants are being developed, alum may remain as an ingredient of adjuvant combinations, or it may eventually be supplemented by other agents that more effectively provide depot and local inflammatory responses to accentuate host immune responses.
Is aluminum sulfate a hazardous substance?
Aluminum sulfate is designated as a hazardous substance under section 311 (b) (2) (A) of the Federal Water Pollution Control Act and further regulated by the Clean Water Act Amendments of 1977 and 1978. These regulations apply to discharges of this substance. This designation includes any isomers and hydrates, as well as any solutions and mixtures containing this substance.
Is aluminum sulfate a caustic?
Aluminum sulfate has an action similar to that of alum but is more stringent. A saturated solution is employed as a mild caustic. Solutions containing 5 to 10% have been used as local applications to ulcers and to arrest foul discharges from mucous surfaces. Aluminium sulfate is also used in the preparation of aluminium acetate ear drops.
Is aluminum sulfate soluble in water?
Aluminum sulfate is also obtained as an 18-hydrate Al2 (SO4)3.18H2O. Both forms are soluble in water, noncombustible, and nontoxic. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment.
Is aluminum sulfate safe for animals?
Aluminum sulfate used as a general purpose food additive in animal drugs, feeds, and related products is generally recognized as safe when used in accordance with good manufacturing or feeding practice. 21 CFR 582.1125; U.S. National Archives and Records Administration's Electronic Code of Federal Regulations.