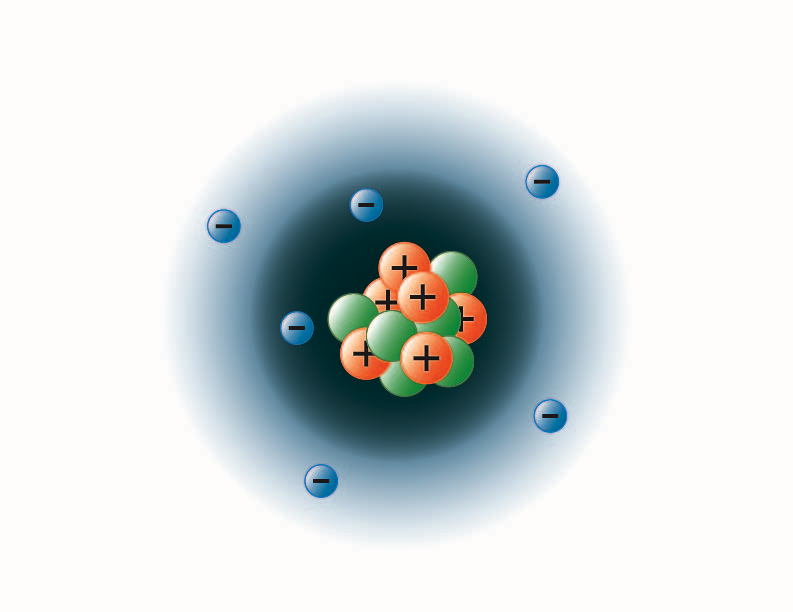
What are the maximum number of electrons in each shell?
Electrons are arranged in different shells around the nucleus. Each successive shell can only hold a certain number of electrons. The innermost shell is filled first. This shell can contain a maximum of two electrons. The second shell can hold a maximum of eight electrons.
How many electrons are in the outermost shell of sodium?
The outermost shell of the sodium ion is the second electron shell, which has eight electrons in it. What is the number of electrons gained or lost in chlorine? Since it has 1 more proton than electrons, sodium has a charge of +1, making it a positive ion. Chlorine gains an electron, leaving it with 17 protons and 18 electrons.
How to determine the number of electron shells?
Thus, group number is a good predictor of how reactive each element will be:
- Helium ( ), neon ( ), and argon ( ), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. ...
- Hydrogen ( ), lithium ( ), and sodium ( ), as group 1 elements, have just one electron in their outermost shells. ...
- Fluorine () and chlorine ( ), as group 17 elements, have seven electrons in their outermost shells. ...
Which elements have two electron shells?
- Hydrogen has 1 electron in the first shell (so one valence electron).
- Helium has 2 electrons --- both in the first shell (so two valence electrons).
- Lithium has 3 electrons --- 2 in the first shell, and 1 in the second shell (so one valence electron).
How many electrons can a shell hold?
What are the electrons in the shells of an atom?
How many electrons go in each shell of sodium?
List of elements with electrons per shellZElementNo. of electrons/shell8Oxygen2, 69Fluorine2, 710Neon2, 811Sodium2, 8, 169 more rows
How many electrons will go in the third shell of sodium?
There is only one electron in the third shell of a neutral sodium atom.
How many electrons can the first shell fill?
2Search formShellSubshellTotal Number of Electrons in Shell1st Shell1s22nd Shell2s, 2p2 + 6 = 83rd Shell3s, 3p, 3d2 + 6 + 10 = 184th Shell4s, 4p, 4d, 4f2 + 6 + 10 + 14 = 32
How many electrons does sodium need to fill its outer shell?
Cations. A neutral sodium atom is likely to achieve an octet in its outermost shell by losing its one valence electron. The cation produced in this way, Na+, is called the sodium ion to distinguish it from the element. The outermost shell of the sodium ion is the second electron shell, which has eight electrons in it.Feb 11, 2019
How many electrons does Na 1 have?
A neutral atom, Na, would have 11 electrons. But this is Na+1. The "+1" tells us that it is missing one electron. The positive charge results from there being more protons than electrons.Sep 16, 2020
How many electrons are there in the third shell of sodium quizlet?
- This is explained by effective nuclear charge. - Ex: Sodium (Na) atomic number 11, the charge on sodiums nucleus is +11. Sodium has three shells of electrons (1s2,2s2,2p6,3s1). So 2 electrons in the first shell, 8 electrons in the second shell, and 1 electrons in the third shell.
Does the third shell have 8 or 18 electrons?
1 Answer. The third shell in its lowest state has room for 8 electrons but including the higher energy 3d electrons it has a capacity of 18 electrons.Oct 22, 2016
Why are there only 2 electrons in the first shell?
There are at most two electrons in the first shell because of the Pauli Exclusion Principle, which says there can be only one electron with a given set of quantum values: only the spin can change, it can be -1/2 or +1/2. So that is two.
How do you calculate electron shells?
0:444:41Electron shells Elements 1-18 - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou can use the periodic table for the groups 1 2 13 14 15 16 17 and 18 in order to find the numberMoreYou can use the periodic table for the groups 1 2 13 14 15 16 17 and 18 in order to find the number of valence electrons in the outer shell. With the exception of helium.
How many electrons are in the outer shell?
eight electronsThe outermost shell in an atom cannot have more than eight electrons even if it has a capacity to take up more electrons. This is also called the octet rule....How are Electrons Distributed in Different Orbits(Shells)Electron ShellMaximum CapacityL Shell8 electronsM Shell18 electronsN Shell32 electrons1 more row•Feb 18, 2016
How many shells are in a sodium ion?
Textbook solution. Na+ ion consist of 2 shells as it consists of 11 electrons and 1 electron is lost from its outer most shell to acquire positive charge. As we know that the atomic number of the sodium (Na+) ion is 11 which mean it has 11 electrons in it.Aug 16, 2019
How many valence electrons does the sodium ion Na 1 have?
The electron configuration shows that the sodium atom has acquired the electron configuration of neon. That is, in this case, the valency of the sodium-ion is +1. Since the last shell of a sodium-ion has eight electrons, the valence electrons of sodium ion(Na+) are eight.
How many electrons can a shell hold?
Shell number one can only hold 2 electrons, shell two can hold 8, and for the first eighteen elements shell three can hold a maximum of eight electrons. As you learn about elements with more than eighteen electrons you will find that shell three can hold more than eight.
What are the electrons in the shells of an atom?
Take a look at the picture below. Each of those colored balls is an electron. In an atom, the electrons spin around the center, also called the nucleus. The electrons like to be in separate shells/orbitals.