How many protons and electrons are present in O2?
Mar 19, 2022 · A neutral oxygen atom as also has 8 electrons. The oxide anion has a charge of 2− . This means that it has gained two electrons from another element, such as sodium or magnesium. All oxide anions, O2− , have 8 protons and 10 electrons.
Can oxygen accept and donate electrons?
Apr 06, 2020 · A neutral oxygen atom as also has 8 electrons. The oxide anion has a charge of 2− . This means that it has gained two electrons from another element, such as sodium or magnesium. All oxide anions, O2− , have 8 protons and 10 electrons.
What element has 4 electrons?
Oct 20, 2021 · A neutral oxygen atom as also has 8 electrons. The oxide anionhas a charge of 2− . This method that that has acquired two electronsfrom an additional element, such as sodium or magnesium. Every oxide anions, O2− , have actually 8 protons and also 10 electrons.
Why is oxygen the final electron acceptor?
The two gained electrons (purple dots) means that this oxygen ion has 10 electrons (-10 charge) and only 8 protons (+8 charge), giving the ion a net charge of -2. Symbolically, we can represent this oxygen ion as O-2. What happens when oxygen gains 2 electrons? Oxygen, O. Oxygen is in Group 6. It has six electrons in its outer shell.
See more
Oct 24, 2009 · There are 8 electrons in the oxygen atom but as an oxygen ion is O 2- then there are 10 electrons in an oxygen ion. How many electrons …
How many electrons are there in O2 ion?
10This means that the nuclei of all oxygen atoms have eight protons. A neutral oxygen atom will also have eight electrons. In an oxygen ion with a 2− charge, the number of protons is still 8, but the number of electrons is 10.30-Dec-2016
How many protons are in O2 ion?
8 protonsA neutral oxygen atom as also has 8 electrons. The oxide anion has a charge of 2− . This means that it has gained two electrons from another element, such as sodium or magnesium. All oxide anions, O2− , have 8 protons and 10 electrons.13-Feb-2018
How many neutrons are in O2 ion?
8 neutronsHow many protons, neutrons, and electrons in a single oxide (O2−) ion? Oxygen has the atomic number 8 so both the atom and the ion will have 8 protons. The average atomic mass of oxygen is 16. Therefore, there will be 8 neutrons (atomic mass−atomic number=neutrons).13-Aug-2020
What is electron of oxygen?
Oxygen Atomic and Orbital PropertiesAtomic Number8Mass Number16Number of Neutrons8Shell structure (Electrons per energy level)[2, 6]Electron Configuration[He] 2s2 2p45 more rows
What are the valence electrons of oxygen (O)?
Oxygen is a non-metallic element. Oxygen is an element of group-16. The valence electron is the total number of electrons in the last orbit. The total number of electrons in the last shell after the electron configuration of oxygen is called the valence electrons of oxygen.
How many electrons and protons does the oxygen atom have?
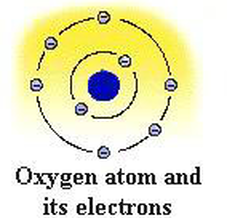
The nucleus is located in the center of the atom. Protons and neutrons are located in the nucleus. The atomic number of oxygen is 8. The atomic number is the number of protons. That is, the number of protons in the oxygen is 8. Electrons equal to protons are located in a circular shell outside the nucleus.
How to determine the valence electron of oxygen?
Now we will know how to easily determine the valence electron of oxygen. The valence electron has to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron without electron configuration.
Determination of the valency of oxygen
The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). The number of unpaired electrons in the last orbit of an element is the valency (valence) of that element. The electron configuration of oxygen in excited state is O* (8) = 1s 2 2s 2 2p x2 2p y1 2p z1.
How many valence electrons does oxygen ion have?
After the electron configuration, the last shell of the oxygen atom has six electrons. After arranging the electrons, it is seen that the last shell of the oxygen atom has six electrons. In this case, the valence electrons of oxygen are 6. We know the details about this.
Compound formation of oxygen by valence electrons
Oxygen participates in the formation of bonds through its valence electrons. We know that the valence electrons in oxygen are six. This valence electron participates in the formation of bonds with atoms of other elements. Oxygen atoms form bonds by sharing electrons with hydrogen atoms.