Orbitals and Electron Capacity of the First Four Principle Energy Levels
| Principle energy level (n) | Type of sublevel | Maximum number of electrons (2n2) |
| 3 | s | 18 |
| 3 | p | 18 |
| 3 | d | 18 |
How many sublevels of electrons can N3 have?
30/03/2020 · Answer and Explanation: The maximum number of electrons that can be contained in the n=3 level is 18. This electron shell has enough energy to contain three sublevels: s, p, Popular
How many electrons can share the quantum number n 2?
3 rows · 03/11/2021 · The maximum number of electrons that can be contained in the n=3 level is 18. This electron ...
How many electrons are in the 2nd energy level?
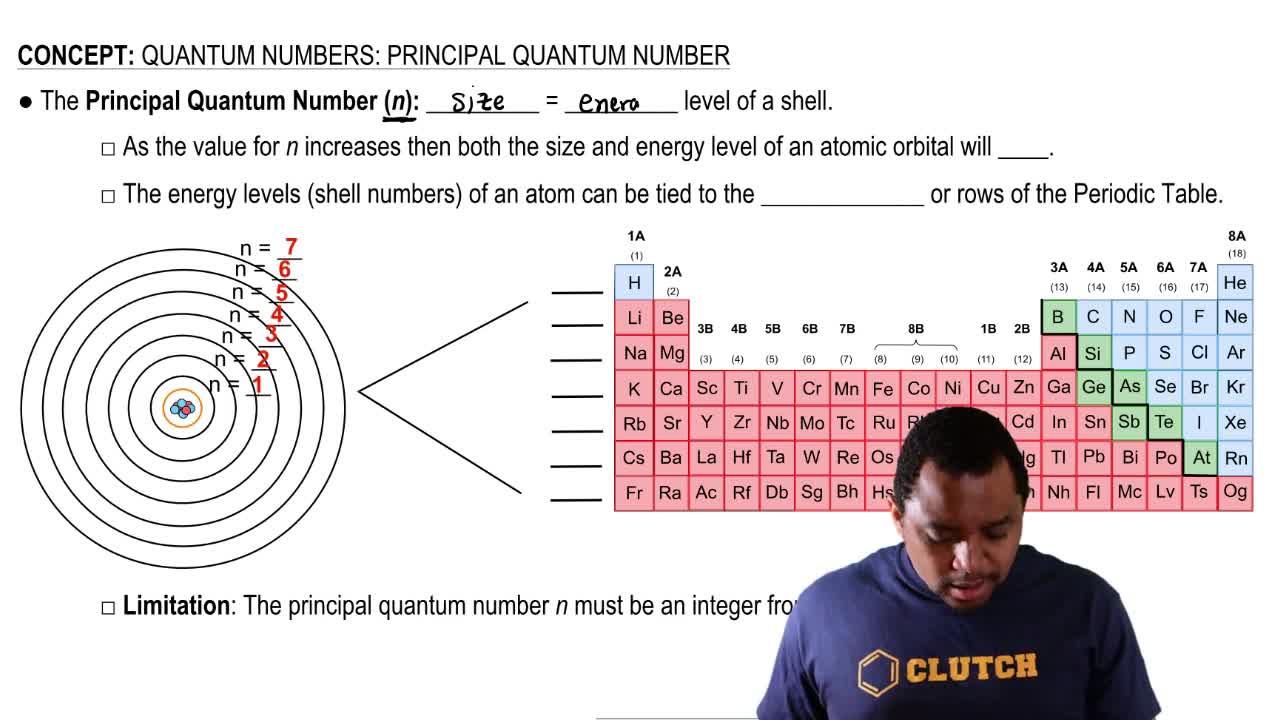
06/04/2018 · How many orbitals are in the n = 3 level? What is the maximum number of electrons in the n = 3 level? Learn this topic by watching Quantum Numbers: Principal Quantum Number Concept Videos
How many electrons are in the 3d subshell?
18/09/2021 · Consider the n 3 energy level in a hydrogen atom. Also in green the position probability distribution w. Answer18 electronsExplanationThere can be up to 2 electrons in 1 orbitalIn the level 3 there are 3 sublevels. These dashed lines represent the different energy levels the electron can have while in the atom.
How many electrons can n 3 hold?
In the n = 3 shell, 2 electrons max fit into the s subshell and 6 electrons max in the 3px, 3py, and 3pz. The maximum number of electrons that can fit inside a 'n' shell is 2n^2. So it is 18. The principle quantum number 'n' defines the shell and not the orbital.
How many orbitals are there in n 3?
nine orbitalsThere are nine orbitals in the n = 3 shell. There is one orbital in the 3s subshell and three orbitals in the 3p subshell.
How many electrons are there in the third shell principal energy level N 3 of the atom with atomic number 23?
There are 13 electrons present in the third shell (principal energy level) of the atom with atomic number 23. Explanation: Number of shells present in an element are K, L, M, N and so on.22-May-2019
How many electrons are there in the 3rd principal energy level N 3 of a phosphorus atom?
Phosphorus has a partially filled 3rd principal energy level with 2 electrons in the 3s and one in the 3p orbital. So the answer is 8.
What is the quantum number of an electron?
The principal quantum number, n, tells you the energy level on which an electron resides. In order to be able to determine how many electrons can share this value of n, you need to determine exactly how many orbitals you have in this energy level.
How many electrons can an orbital hold?
no. of orbitals = n2. Since each orbital can hold a maximum of two electrons, it follows that as many as. no. of electrons = 2n2. In this case, the second energy level holds a total of. no. of orbitals = n2 = 22 = 4. orbitals. Therefore, a maximum of.
How many orbitals can a subshell hold?
Now, the number of orbitals you get per subshell is given by the magnetic quantum number, ml, which in this case can be. So, the f-subshell can hold total of seven orbitals, which means that you have a maximum of. electrons that can share these two quantum numbers, n = 4 and l = 3.