When NaOH reacts with an acid, it accepts the proton lost by the acid and behaves like a Bronsted-Lowry base. In NaOH, OH – accepts the proton. Thus, a higher concentration of hydroxide ions means a stronger base.
Is NaOH regarded as a Bronsted base?
Is NaOH a Bronsted base? NaOH is a Brønsted-Lowry base because it accepts H+ ions from acids. NaOH is a Lewis base because the lone pairs on the hydroxide ion can be donated to other compounds. Is NaOH a Arrhenius base? NaOH is an Arrhenius base because it dissociates in water to give the hydroxide (OH-) and sodium (Na+) ions.
Why is NaOH considered a strong base?
What type of base is NaOH? Strong bases are characterized by the fact that they dissociate completely in aqueous solution. In this case, sodium hydroxide, NaOH , is classified as a strong base because it dissociates completely in aqueous solution to form sodium cations, Na+ , and hydroxide anions, OH− . Is KBr an acid or base?
Is NaOH a strong or weak acid or base?
Sodium hydroxide (NaOH) is strong base because it fully dissociates in water to produce hydroxide ions. While ammonia (NH3) is weak base because it accepts protons from water to produce fewer hydroxide ions in solution. Also Know, is h2co3 a strong or weak acid or base? NaOH is a strong base that completely dissociates in water.
Is LiOH a weaker base than NaOH?
No, LiOH is a strong base, like the other alkali bases. It is a bit weaker than bases like NaOH though, because it’s enthalpy of formation is different. Its conjugate acid, Li+, can act as a weak Lewis acid.
See more
Is NaOH a strong Brønsted-Lowry base?
NaOH acts as Arrhenius base and Bronsted-Lowry base. NaOH act as a Lewis base. it has an OH-- ion that can donate the electron pair to another compound acceptor. NaOH is the strong base.
Is hydroxide a Brønsted-Lowry base?
The ammonium ion is a Brønsted-Lowry acid, while the hydroxide ion is a Brønsted-Lowry base.
Is NaOH an Arrhenius or a Brønsted base?
Solution: The Brønsted-Lowry definition says that a base accepts protons (H+ ions). NaOH, Ca(OH)2, and KOH are all Arrhenius bases because they yield the hydroxide ion (OH-) when they ionize.
How do you know if it is a Brønsted-Lowry base?
4:099:39Identifying Bronsted Lowry Acids and Bases - Real Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipHere's the trick if you have a bronsted-lowry acid. After it donates a hydrogen it becomes aMoreHere's the trick if you have a bronsted-lowry acid. After it donates a hydrogen it becomes a conjugate base so that means fluorine is a conjugate base.
What is an example of a Bronsted-Lowry base?
Water is the Bronsted-Lowry base because it is the 'proton acceptor' - it accepted a hydrogen atom from sulfuric acid (H sub 2 SO sub 4). Meanwhile, we identify sulfuric acid as the Bronsted-Lowry acid because it donated a proton to water.
Is sodium hydroxide a base?
General information. Sodium hydroxide (caustic soda) is highly soluble in water, and sodium hydroxide solutions are strong bases.
What type of base is NaOH?
Sodium hydroxide (Na OH), also known as lye or caustic soda, is a caustic metallic base. An alkali, caustic soda is widely used in many industries, mostly as a strong chemical base in the manufacture of pulp and paper, textiles, drinking water, and detergents.
How is NaOH an Arrhenius base?
NaOH is an Arrhenius base because it dissociates in water to give the hydroxide (OH-) and sodium (Na+) ions. An Arrhenius acid is therefore any substance that ionizes when it dissolves in water to give the H+, or hydrogen, ion.
Why is NaOH basic?
Sodium hydroxide (NaOH) is strong base because it fully dissociates in water to produce hydroxide ions. While ammonia (NH3) is weak base because it accepts protons from water to produce fewer hydroxide ions in solution.
How do you know if a compound is a Brønsted-Lowry acid?
A Brønsted-Lowry base is any species that can accept a proton from another molecule. In short, a Brønsted-Lowry acid is a proton donor (PD), while a Brønsted-Lowry base is a proton acceptor (PA). Thus H+ is an acid by both definitions, and OH− is a base by both definitions.
What makes something a Brønsted-Lowry acid?
A Bronsted-Lowry acid is defined as a substance that gives up or donates hydrogen ions during a chemical reaction. In contrast, aBronsted-Lowry base accepts hydrogen ions. Another way of looking at it is that a Bronsted-Lowry acid donates protons, while the base accepts protons.
Which acts a Bronsted acid and Brønsted base?
The species: H2O,HCO−3,HSO−4 and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
Does Bronsted Lowry go against Arrhenius?
The Bronsted-Lowry theory doesn't go against the Arrhenius theory in any way - it just adds to it.
Is ammonia an Arrhenius base?
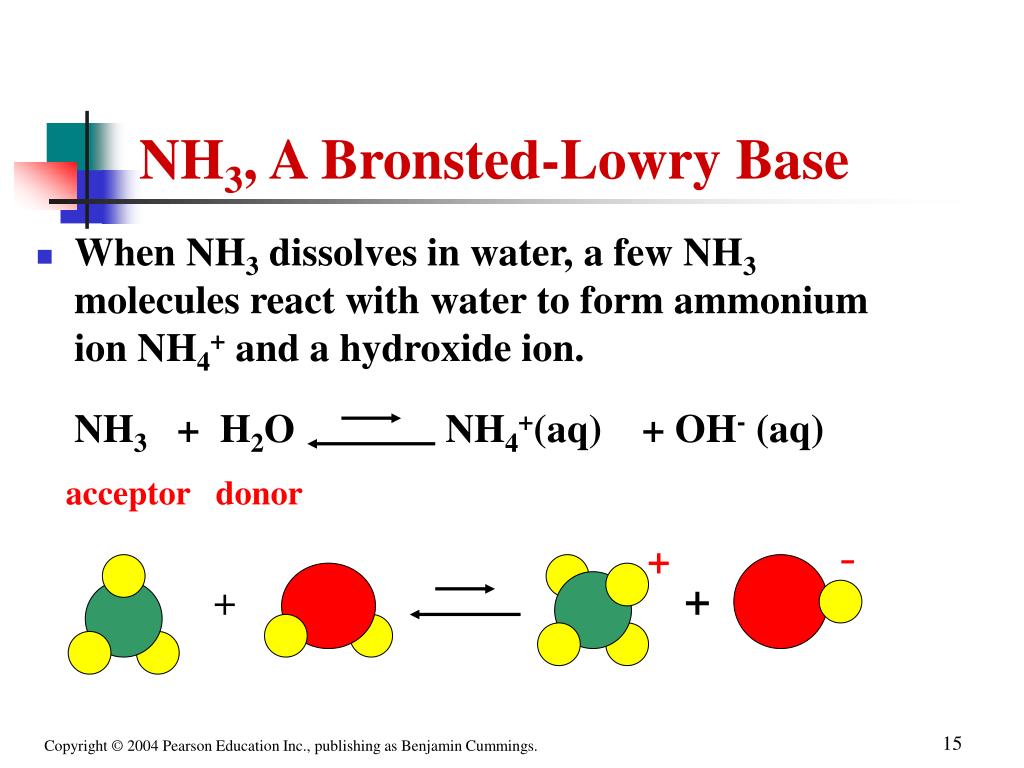
Well, let's take very simple Brønsted-Lowry base $ce{NH3}$, as it accepts a $ce{H+}$in acidic medium or in aqueous medium, so we call ammonia as a Brønsted-Lowry base, whereas $ce{NaOH}$(sodium hydroxide) is an Arrhenius base, as $ce{OH-}$from $ce{NaOH}$will accept $ce{H+}$, but according to Brønsted-Lowry theory, in this case hydroxide ionshould be the Brønsted-Lowry base not sodium hydroxide.
Is Arrhenius acid a Bronsted Lowry base?
All Arrhenius acids and bases are also Bronsted- Lowry bases. According to this website, The Bronsted-Lowry theory doesn't go against the Arrhenius theory in any way - it just adds to it. Remember, when we write NaOH (aq), what we really mean is Na+ (aq) and OH- (aq). Sodium hydroxide is a Bronsted-Lowry base because the hydroxide ions ...
Is NaOH a base or a compound?
NaOH, or sodium hydroxide, is a compound. A compound is classified as either an acid, base, or salt. All bases contain OH- (hydroxide) ions, while all acids contain H+ (hydrogen) ions.
Is NaOH a base or an acid?
NaOH is a base because when dissolved in water it dissociates into Na+ and OH- ions. In terms of Lewis theory a base is defined as a compound which donates a pair of electrons and an acid is defined as the one which accept NaOH is a base because when dissolved in water it dissociates into Na+ and OH- ions.
Why is NaOH a Base?
Different scientists gave different definitions for acids and bases. We will focus on three main theories.
What makes NaOH a Strong Base?
A base can be classified as strong or weak (according to Arrhenius theory) depending on the extent of hydroxide ions released on dissolving in water.
What Factors affect the Strength of a Base?
1. Atomic Size: A large atom with high polarizability can donate electron pairs easily and is more basic than a smaller atom.