The more salt there is dissolved in the water, the greater its salinity. When comparing two samples of water with the same volume, the water sample with higher salinity will have greater mass, and it will therefore be more dense. The density of water can also be affected by temperature.
What happens to the density of water when salinity increases?
Salinity can affect the density of ocean water: Water that has higher salinity is denser and heavier and will sink underneath less saline, warmer water. This can affect the movement of ocean currents. It can also affect marine life, which may need to regulate its intake of saltwater.Dec 9, 2019.
What decreases the salinity of water?
Density of Ocean water | UPSC – IAS
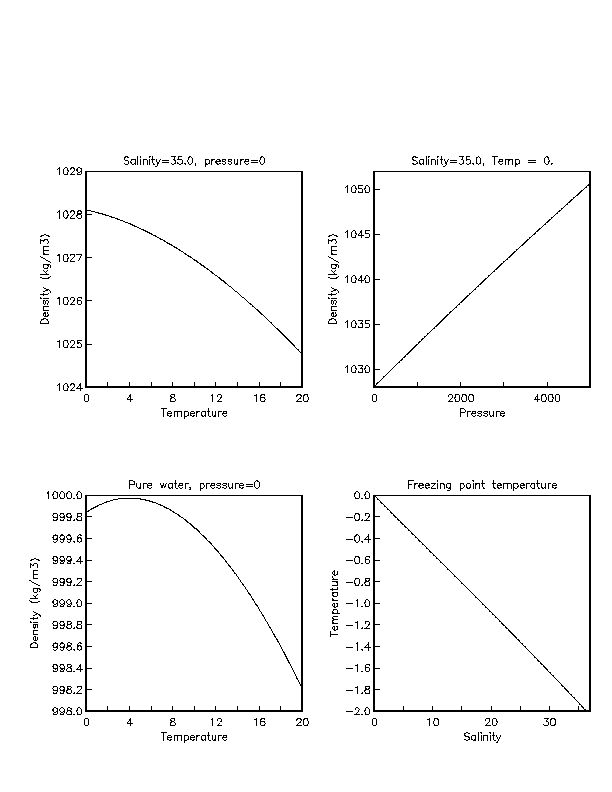
- The density of any substances is the mass per unit volume stated in grams per cubic centimeter. ...
- Pure water has maximum density of one unit at temperature 4 degree Celsius, whereas for the sea it changes according to the salinity content.
- Density of pure water depends upon temperature & pressure only.
How is salinity related to the density of water?
- In organisms generally, with particular emphasis on human health Electrolytes Fluid balance Hypernatremia Hyponatremia Salt poisoning
- In plants Arabidopsis thaliana responses to salinity
- In fish Stenohaline fish Euryhaline fish
What affects density more temperature or salinity?
Temperature has a greater effect on the density of water than salinity does. So a layer of water with higher salinity can actual float on top of water with lower salinity if the layer with higher salinity is quite a bit warmer than the lower salinity layer. Click to read full detail here. Also question is, how does temperature affect salinity?
How does salinity affect water density?
The more salt there is dissolved in the water, the greater its salinity. When comparing two samples of water with the same volume, the water sample with higher salinity will have greater mass, and it will therefore be more dense.
What happens when you add salt to water experiment?
When you add salt to the water, you increase the density. That is to say, the salt packs into the same volume of water. With enough salt added to the water, the density of the water is greater than the egg, allowing the egg to float.
How do you do a salt water density experiment?
0:071:11Salt Water Density Experiment | Daycare Activities - YouTubeYouTubeStart of suggested clipEnd of suggested clipYou can play around with the ratios of sugar and salt in each cup as well. Once it's completelyMoreYou can play around with the ratios of sugar and salt in each cup as well. Once it's completely stirred up we are going to label all of our cups using sticky notes and a marker.
What is the independent variable in salt water experiment?
The independent variable is the salt added in the water, whereas the dependent variable is the boiling point of the water.
What is the relationship between density and salinity?
The density of water increases as the salinity increases. The density of seawater (salinity greater than 24.7) increases as temperature decreases at all temperatures above the freezing point.
Why is salt water more dense?
The h20 molecules cluster around the salt molecules, and the result is that saltwater has more molecules overall than freshwater. When you've added more weight to that cubic foot of water (the salt), you are producing a denser type of water.
Why does salt make things float in water?
It has to do with the density of the objects compared with the density of the water surrounding them. If an object is less dense than the water around it, it will float. Because salt water is denser than freshwater, some things float more easily in the ocean—or extremely salty bodies of the water, such as the Dead Sea.
Why does salt water help you float?
The reason is: Buoyancy – saltwater gives more buoyancy than freshwater because of the higher density of saltwater. Buoyancy makes it easy for the body to stay high in water, thus all other factors being kept equal, one can swim faster in salt water than in freshwater.
How does the addition of salt affect the density of water quizlet?
What are the effects of adding salts to water? It increases the density of water.
Is water salinity a dependent variable?
VARIABLES: The independent variable is the different amounts salt that will be added to the water (what is done by the scientist). The dependent variable is the boiling temperature of the water (what is being measured). III: HYPOTHESIS: If salt is added to water then, the boiling temperature is lowered.
What are the independent and dependent variables in this experiment?
Independent variables (IV): These are the factors or conditions that you manipulate in an experiment. Your hypothesis is that this variable causes a direct effect on the dependent variable. Dependent variables (DV): These are the factor that you observe or measure.
How does salt affect the boiling point of water experiment results?
It was found that adding salt to water increases the boiling time of water. The more salt you add, the higher the boiling temperature becomes therefore the solution takes a longer period of time to boil.
What does the water density experiment show?
The water density experiment demonstrates how salinity and temperature effect water density.
How to make salinity solution?
Create various salinity solutions and color code them with food coloring: Mix 4 tablespoons of salt into first cup, then add 2 drops of red food coloring. Mix 2 tablespoons of salt into second cup , ...
How much water can a glass jar hold?
Glass jar or similar transparent container large enough to hold 3 cups of water (3 per student or group)
What are the parts of the water density experiment?
This experiment contains 2 parts: Part 1 tests the effect of temperature on water density. Part 2 tests the effect of salinity on water density.
Is it appropriate to implement a science project idea?
Warning is hereby given that not all Project Ideas are appropriate for all individuals or in all circumstances. Implementation of any Science Project Idea should be undertaken only in appropriate settings and with appropriate parental or other supervision. Reading and following the safety precautions of all materials used in a project is the sole responsibility of each individual. For further information, consult your state's handbook of Science Safety.
Can you add salt to water?
Do not add salt to the water. First pour the saltiest water (red) into the last unused glass jar, next pour the slightly salty water (yellow) slowly into glass jar, and finally pour the pure water (blue) slowly into the glass jar. Students should record observations of which levels of salinity sink/float compared to the others.
Is it appropriate to implement a science project idea?
Warning is hereby given that not all Project Ideas are appropriate for all individuals or in all circumstances. Implementation of any Science Project Idea should be undertaken only in appropriate settings and with appropriate parental or other supervision. Reading and following the safety precautions of all materials used in a project is the sole responsibility of each individual. For further information, consult your state's handbook of Science Safety.
Is cold water denser than warm water?
Cold water is denser than warm water. Water with a high salt concentration ( salinity) is denser than water that has a lower salt concentration. Warm water rises above denser colder water, and bodies of water that have different temperatures can form layers according to their respective temperatures. Likewise, denser water with high salinity sinks ...
How to make fresh water more dense than egg?
In the same jar, start adding large scoops of salt into the water. You may need to stir the salt around to ensure it dissolves into the water. Keep adding salt until the egg floats to the top. When this happens, the water is now more dense than the egg. Next, let’s see what happens when fresh water and salt water meet.
Why doesn't water mix in jars?
Notice how the colours contained within each jar of water don’t mix: this is because the “fresh” water in in the second jar is less dense than the salty water in the first. In nature, this effect is known as a “halocline”.
How to make a salt jar?
To the first jar on your plastic tray, add a spoonful of salt and stir. Place the plastic board on top of the second jar, and hold tight to flip the jar over. With the board still in place underneath the second jar, place it on top of the first jar and ensure the openings of both jars are aligned.
Is fresh water less dense than salt water?
Evan is back with another impressive water experiment that you can try at home to learn about water salinity and density. Fresh water is less dense than salt water, so it can sit on top. In undistributed waters, they can remain as separate bodies for a long time! This is referred to as a salt wedge. Did you know there is a pretty amazing salt wedge ...
Why does salt make water denser?
Adding salt to water makes the water denser. As the salt dissolves in the water, it adds mass (more weight to the water). This makes the water denser and allows more objects to float on the surface that would sink in fresh water.
What percentage of seawater comes from salt?
About 3.5 percent of the weight of seawater comes from the dissolved salts.
What are the two most common ions in seawater?
Two of the most common ions in seawater are chloride and sodium. Together, they make up over 90 percent of all dissolved ions in the ocean. Sodium and Chloride are ‘salty.’. In fact, sodium chloride is the chemical name for salt!
What happens when rain erodes rocks?
As the rain erodes the rock, acids in the rainwater break down the rock. This process creates ions, or electrically charged atomic particles. These ions are carried away in runoff to streams and rivers and, ultimately, to the ocean.
Why do we float better in salt water?
So, why do we, and the egg, float better in a salt water solution? The answer is a physical property called density. Density is the amount of mass contained per unit volume. The more mass that fits in a space, the greater the density.
Why did the egg in the salt water sunk?
But, because the egg has a density greater than water alone , the egg in the plain water sunk.
How to make a salt solution for eggs?
First, prepare your salt solution. Fill two glasses with warm tap water (warm water will help dissolve the salt better) about 3/4 full or enough to completely submerge your egg. Mix 3 tablespoons of salt into one glass. Leave the other glass with plain water.
What happens if you don't dissolve salt?
If it isn't, your salt solution concentration may not be correct and will affect the floatation of your egg.
When an object is in water, does it sink?
When an object is in water, if the density is greater than water, it will sink. However, if the density of the object is less than water, it will float.
How to stay afloat in the ocean?
Have you ever been to the beach? You can nearly taste the salt water in the air as the warm breeze blows around you. If you've ever gone for a swim in the ocean, you know how easy it is to stay afloat. Simply laying on your back lets you relax effortlessly in the waves.
What is the difference between salinity and temperature?
In seawater, the main chemical is sodium chloride (salt), but there are many others in smaller quantities. temperature: A measure of the degree of hotness or coldness of an object or substance.
Which is denser, cold or fresh water?
Cold water is denser than warm water, so it tends to sink. Seawater is denser than freshwater. Salinity. 1. , temperature. 2. and depth all affect the density. 3.
How does the ocean circulate?
The ocean has a complex circulation system called the Global Ocean Conveyor. It moves water, heat, salt and nutrients around the world. Surface currents in the top 400 m are driven mainly by wind. Deeper currents are driven by changes in water density. Both types of currents work with the atmosphere to help shape the Earth’s climate.
What happens to the density of salt when it is dissolved in fresh water?
When salt is dissolved in fresh water, the density of the water increases because the mass of the water increases. This is represented by the addition of red spheres and blue cubes to the box from Fig. 2.2 A to Fig. 2.2 D. Salinity describes how much salt is dissolved in a sample of water. The more salt there is dissolved in the water, ...
How does temperature affect water density?
The density of water can also be affected by temperature. When the same amount of water is heated or cooled, its density changes. When the water is heated, it expands, increasing in volume. This is represented by the increase in the size of the box from Fig. 2.2 A to 2.2 C. The warmer the water, the more space it takes up, and the lower its density. When comparing two samples of water with the same salinity, or mass, the water sample with the higher temperature will have a greater volume, and it will therefore be less dense.
What happens when the density of a cube is less than the density of the water?
The density of the cube relative to the density of water determines if the cube will float, sink, or be neutrally buoyant: If the density of the cube is less than the density of the water, gravitational force will be less than the buoyant force (G < B), and the object will rise to the surface (Fig. 2.6 A).
Why does a cube float in the middle of the column of water?
If the density of the cube is equal to the density of the water, the cube will float in middle of the column of water because the gravitational force and buoyant force are balanced (G = B). This cube is neutrally buoyant (Fig. 2.6 B).
What happens when you compare two samples of water with the same volume?
When comparing two samples of water with the same volume, the water sample with higher salinity will have greater mass, and it will therefore be more dense.
Why do water layers form?
If water masses have salinity or temperature differences , they will form water layers because they have different densities . Water layers can sometimes be felt when swimming. For example, on hot days the sun’s heat can make water at the surface noticeably warmer than the deeper, cooler water. The relative density of one water mass in relation to another determines whether a layer of water floats or sinks.
Why does surface floating occur?
Surface floating occurs when an object stays at the surface, because the forces are balanced at the surface (G = B).
How does salt change the density of water?
Adding salt to water makes the water denser. As the salt dissolves in the water, it adds mass (more weight to the water). This makes the water denser and thus allows more objects to float on the surface that would sink in fresh water.
What happens when you add salt to water?
When added to water, objects that are denser than water sink and those that are less dense than water float. Hollow things often float too as air is less dense than water. You can experiment with many objects that sink and float in water, but what happens when you add salt to the water?
How to make a salt water egg?
STEP 1: Start by filling one glass about 2/3 of the way full with water. Ask the kids what will happen if you carefully drop an egg into the glass of water. Now go ahead and do it! STEP 2: In the other glass, fill to the same height with water. Now stir in 3 tablespoons of salt. Mix well to dissolve the salt!
Why does the second egg float?
The second egg should float due to the change in density of the water!
What will work best with the measurements of salt and water provided?
Small plastic items will work best with the measurements of salt and water provided. If the item still sinks in the saltwater, ask the kids what they think! Should they add more salt? Have each kid contribute an item to the experiment!
Why is the ocean salty?
Why is the ocean salty? The simple answer is that the salt comes from the rocks on the land that has been broken down by erosion and is carries by streams to the ocean.
Do objects float better in saltwater or freshwater?
What other items can you find to test? In general, most items will float in this saltwater experiment even if they sink in freshwater. Just look at the egg!